Pharmacokinetics, metabolism and serum concentrations of progestins used in contraception
- PMID: 33316287
- PMCID: PMC8122039
- DOI: 10.1016/j.pharmthera.2020.107789
Pharmacokinetics, metabolism and serum concentrations of progestins used in contraception
Abstract
Many different forms of hormonal contraception are used by millions of women worldwide. These contraceptives differ in the dose and type of synthetic progestogenic compound (progestin) used, as well as the route of administration and whether or not they contain estrogenic compounds. There is an increasing awareness that different forms of contraception and different progestins have different side-effect profiles, in particular their cardiovascular effects, effects on reproductive cancers and susceptibility to infectious diseases. There is a need to develop new methods to suit different needs and with minimal risks, especially in under-resourced areas. This requires a better understanding of the pharmacokinetics, metabolism, serum and tissue concentrations of progestins used in contraception as well as the biological activities of progestins and their metabolites via steroid receptors. Here we review the current knowledge on these topics and identify the research gaps. We show that there is a paucity of research on most of these topics for most progestins. We find that major impediments to clear conclusions on these topics include a lack of standardized methodologies, comparisons between non-parallel clinical studies and variability of data on serum concentrations between and within studies. The latter is most likely due, at least in part, to differences in intrinsic characteristics of participants. The review highlights the importance of insight on these topics in order to provide the best contraceptive options to women with minimal risks.
Keywords: Contraception; Metabolism; Pharmacokinetics; Progestin; Serum concentration.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that there are no conflicts of interest.
Figures


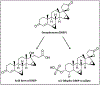

References
-
- Abdalla KA, Shabaan MM, & Stanczyk FZ (1992). Interrelationship of serum levonorgestrel and sex hormone-binding globulin levels following vaginal and oral administration of combined steroid contraceptive tablets. Contraception, 45, 111–118. - PubMed
-
- Abujrais S, Olovsson M, Ahnoff M, Rasmusson AJ, Larsson A, Akerfeldt T, et al. (2019). A sensitive method detecting trace levels of levonorgestrel using LC-HRMS. Contraception, 100, 247–249. - PubMed
-
- Achilles SL, Hendrix CW, & Poloyac SM (2018). Safety and pharmacokinetics of dapivirine and levonorgestrel vaginal rings for multipurpose prevention of HIV and pregnancy. In HIV Research for Prevention (HIVR4P). Madrid.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

