Does Transfusion of Red Blood Cells Impact Germline Genetic Test Results?
- PMID: 33316904
- PMCID: PMC7768420
- DOI: 10.3390/jpm10040268
Does Transfusion of Red Blood Cells Impact Germline Genetic Test Results?
Abstract
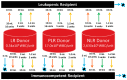
Purpose: molecular testing is often indicated for recently transfused patients. However, there are no guidelines regarding the potential interference from donor DNA or whether it is necessary to wait for a period of time post-transfusion prior to genetic testing. While the majority of patients are transfused in the non-trauma setting using leukoreduced (LR) red blood cell products, the degree of leukoreduction varies among centers and is not universally practiced.
Methods: whole blood units collected from anonymous donors were used in an in vitro transfusion model. One unit was split: half being leukoreduced simulating a leukopenic recipient and half left untreated. Donors were simulated by leukoreduced, partially leukoreduced (PLR), or non-leukoreduced units, transfused in 2, 5, or 16 unit equivalents. DNA from the combinations were subjected to short tandem repeat (STR) analysis for chimerism detection.
Results: donor DNA was not detectable in any of the LR combinations, but detected in the PLR combinations, ranging from 0.1 to 1.5% donor DNA in the immunocompetent recipient and 6.3-27.8% in the leukopenic recipient. Non-LR donor DNA was also detected (13-95%).
Conclusion: donor-derived DNA from leukoreduced blood products is unlikely to interfere with the interpretation of germline genetic testing in immunocompetent recipients but may interfere in immunocompromised recipients.
Keywords: genetic testing; interference; leukoreduction; transfusion; transfusion-associated microchimerism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Microchimerism in immune competent patients related to the leukocyte content of transfused red blood cell concentrates.Transfus Apher Sci. 2004 Dec;31(3):173-80. doi: 10.1016/j.transci.2004.07.012. Transfus Apher Sci. 2004. PMID: 15556463
-
Is cytomegalovirus testing of blood products still needed for hematopoietic stem cell transplant recipients in the era of universal leukoreduction?Biol Blood Marrow Transplant. 2013 Dec;19(12):1719-24. doi: 10.1016/j.bbmt.2013.09.013. Epub 2013 Oct 5. Biol Blood Marrow Transplant. 2013. PMID: 24099958
-
The impact of transfused blood products on deceased donor HLA typing.Hum Immunol. 2019 Dec;80(12):976-982. doi: 10.1016/j.humimm.2019.10.001. Epub 2019 Oct 15. Hum Immunol. 2019. PMID: 31627937 Free PMC article.
-
Immune modulation and microchimerism after unmodified versus leukoreduced allogeneic red blood cell transfusion in cancer patients: results of a randomized study.Transfusion. 2007 Sep;47(9):1691-9. doi: 10.1111/j.1537-2995.2007.01344.x. Transfusion. 2007. PMID: 17725736 Clinical Trial.
-
[Single-donor (apheresis) platelets and pooled whole-blood-derived platelets--significance and assessment of both blood products].Clin Lab. 2014;60(4):S1-39. doi: 10.7754/clin.lab.2014.140210. Clin Lab. 2014. PMID: 24779310 Review. German.
Cited by
-
An Ambiguous NUDT15 Signal in a Child with B-cell Acute Lymphoblastic Leukemia.Clin Chem. 2025 Aug 1;71(8):835-839. doi: 10.1093/clinchem/hvaf028. Clin Chem. 2025. PMID: 40746189 No abstract available.
-
Clinical utility of rapid whole genome sequencing in neonatal patients receiving extracorporeal membrane oxygenation (ECMO).J Perinatol. 2025 Apr;45(4):495-499. doi: 10.1038/s41372-024-02181-1. Epub 2024 Nov 27. J Perinatol. 2025. PMID: 39604575
-
[Genotyping and its applications, a look to the future].Rev Med Inst Mex Seguro Soc. 2023 Jan 1;61(Suppl 1):S37-S45. Rev Med Inst Mex Seguro Soc. 2023. PMID: 36378105 Free PMC article. Review. Spanish.
References
LinkOut - more resources
Full Text Sources

