Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study
- PMID: 33317059
- PMCID: PMC7764650
- DOI: 10.3390/jcm9123989
Characterization of a Pan-Immunoglobulin Assay Quantifying Antibodies Directed against the Receptor Binding Domain of the SARS-CoV-2 S1-Subunit of the Spike Protein: A Population-Based Study
Abstract
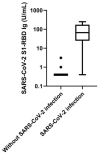
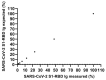
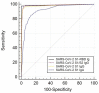
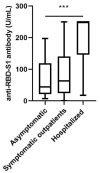
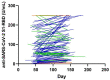
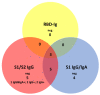
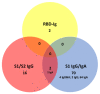
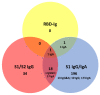
Pan-immunoglobulin assays can simultaneously detect IgG, IgM and IgA directed against the receptor binding domain (RBD) of the S1 subunit of the spike protein (S) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 S1-RBD Ig). In this work, we aim to evaluate a quantitative SARS-CoV-2 S1-RBD Ig electrochemiluminescence immunoassay (ECLIA) regarding analytical, diagnostic, operational and clinical characteristics. Our work takes the form of a population-based study in the principality of Liechtenstein, including 125 cases with clinically well-described and laboratory confirmed SARS-CoV-2 infection and 1159 individuals without evidence of coronavirus disease 2019 (COVID-19). SARS-CoV-2 cases were tested for antibodies in sera taken with a median of 48 days (interquartile range, IQR, 43-52) and 139 days (IQR, 129-144) after symptom onset. Sera were also tested with other assays targeting antibodies against non-RBD-S1 and -S1/S2 epitopes. Sensitivity was 97.6% (95% confidence interval, CI, 93.2-99.1), whereas specificity was 99.8% (95% CI, 99.4-99.9). Antibody levels linearly decreased from hospitalized patients to symptomatic outpatients and SARS-CoV-2 infection without symptoms (p < 0.001). Among cases with SARS-CoV-2 infection, smokers had lower antibody levels than non-smokers (p = 0.04), and patients with fever had higher antibody levels than patients without fever (p = 0.001). Pan-SARS-CoV-2 S1-RBD Ig in SARS-CoV-2 infection cases significantly increased from first to second follow-up (p < 0.001). A substantial proportion of individuals without evidence of past SARS-CoV-2 infection displayed non-S1-RBD antibody reactivities (248/1159, i.e., 21.4%, 95% CI, 19.1-23.4). In conclusion, a quantitative SARS-CoV-2 S1-RBD Ig assay offers favorable and sustained assay characteristics allowing the determination of quantitative associations between clinical characteristics (e.g., disease severity, smoking or fever) and antibody levels. The assay could also help to identify individuals with antibodies of non-S1-RBD specificity with potential clinical cross-reactivity to SARS-CoV-2.
Keywords: COVID-19; SARS-CoV-2; area under the curve; coronavirus; diagnostic accuracy; immunoassay; predictive values; sensitivity; serology; specificity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures
Similar articles
-
Serological testing for SARS-CoV-2 antibodies in clinical practice: A comparative diagnostic accuracy study.Allergy. 2022 Jul;77(7):2090-2103. doi: 10.1111/all.15206. Epub 2022 Jan 11. Allergy. 2022. PMID: 34986501 Free PMC article.
-
Assessment of S1-, S2-, and NCP-Specific IgM, IgA, and IgG Antibody Kinetics in Acute SARS-CoV-2 Infection by a Microarray and Twelve Other Immunoassays.J Clin Microbiol. 2021 Apr 20;59(5):e02890-20. doi: 10.1128/JCM.02890-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33602698 Free PMC article.
-
Analytical characterization of the SARS-CoV-2 EURM-017 reference material.Clin Biochem. 2022 Mar;101:19-25. doi: 10.1016/j.clinbiochem.2021.12.009. Epub 2021 Dec 18. Clin Biochem. 2022. PMID: 34933006 Free PMC article.
-
Response kinetics of different classes of antibodies to SARS-CoV2 infection in the Japanese population: The IgA and IgG titers increased earlier than the IgM titers.Int Immunopharmacol. 2022 Feb;103:108491. doi: 10.1016/j.intimp.2021.108491. Epub 2021 Dec 21. Int Immunopharmacol. 2022. PMID: 34954559 Free PMC article.
-
Antibodies against the SARS-CoV-2 S1-RBD cross-react with dengue virus and hinder dengue pathogenesis.Front Immunol. 2022 Aug 15;13:941923. doi: 10.3389/fimmu.2022.941923. eCollection 2022. Front Immunol. 2022. PMID: 36045680 Free PMC article.
Cited by
-
Serology suggests adequate safety measures to protect healthcare workers from COVID-19 in Shiga Prefecture, Japan.PLoS One. 2022 Jun 24;17(6):e0270334. doi: 10.1371/journal.pone.0270334. eCollection 2022. PLoS One. 2022. PMID: 35749426 Free PMC article.
-
Antibody titres decline 3-month post-vaccination with BNT162b2.Emerg Microbes Infect. 2021 Dec;10(1):1495-1498. doi: 10.1080/22221751.2021.1953403. Emerg Microbes Infect. 2021. PMID: 34232116 Free PMC article.
-
Safety Evaluation of a Medical Congress Held During the COVID-19 Pandemic-A Prospective Cohort.Int J Public Health. 2022 Feb 16;67:1604147. doi: 10.3389/ijph.2022.1604147. eCollection 2022. Int J Public Health. 2022. PMID: 35250429 Free PMC article.
-
Serial Changes of Long COVID Symptoms and Clinical Utility of Serum Antibody Titers for Evaluation of Long COVID.J Clin Med. 2022 Feb 27;11(5):1309. doi: 10.3390/jcm11051309. J Clin Med. 2022. PMID: 35268400 Free PMC article.
-
Evaluations of SARS-CoV-2 Serological Assay Performance Need Inclusion of Long-Term Samples.J Clin Microbiol. 2021 May 19;59(6):e00487-21. doi: 10.1128/JCM.00487-21. Print 2021 May 19. J Clin Microbiol. 2021. PMID: 33758035 Free PMC article. No abstract available.
References
-
- Patel R., Babady N.E., Theel E.S., Storch G.A., Pinsky B.A., George K.S., Smith T.C., Bertuzzi S. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS–CoV-2/COVID-19. mBio. 2020;11 doi: 10.1128/mBio.00722-20. - DOI - PMC - PubMed
-
- Baron R.C., Risch L., Weber M., Thiel S., Grossmann K., Wohlwend N., Lung T., Hillmann D., Ritzler M., Bigler S., et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: A population-based study. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0978. - DOI - PubMed
-
- Thiel S.L., Weber M.C., Risch L., Wohlwend N., Lung T., Hillmann D., Ritzler M., Risch M., Kohler P., Vernazza P., et al. Flattening the curve in 52 days: Characterisation of the COVID-19 pandemic in the Principality of Liechtenstein—An observational study. Swiss Med. Wkly. 2020;150:w2036. doi: 10.4414/smw.2020.20361. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

