Generic and Respiratory-Specific Quality of Life in Non-Hospitalized Patients with COVID-19
- PMID: 33317214
- PMCID: PMC7764406
- DOI: 10.3390/jcm9123993
Generic and Respiratory-Specific Quality of Life in Non-Hospitalized Patients with COVID-19
Abstract
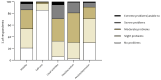
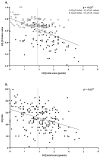
The impact of coronavirus disease 2019 (COVID-19) on quality of life appears to be highly underestimated, especially in patients who have not been admitted to the hospital. Therefore, our aim was to assess respiratory-specific quality of life in addition to generic quality of life in former patients with confirmed/suspected COVID-19 who have never been admitted to the hospital. Members of an online Belgian social support group for patients with confirmed/suspected COVID-19 with persistent complaints, completed an online survey. The five-level EQ-5D (EQ-5D-5L) and the Clinical COPD Questionnaire (CCQ) were used to assess generic and respiratory-specific quality of life, respectively. Data of 210 non-hospitalized patients (88% women, 45 ± 11 years, 79 ± 17 days after symptom onset) were included in the analyses. Mean EQ-5D index and visual analogue scale (EQ-VAS) score was 0.62 ± 0.19 and 50.71 ± 18.87, respectively, with 40% of the patients demonstrating an EQ-5D index that was below the fifth percentile of normative values, indicating poor generic quality of life. The mean CCQ score was 2.01 ± 0.98 points, while 123 respondents (59%) had a total score ≥1.9 points, indicating poor respiratory-specific quality of life. The correlation between EQ-5D index score/EQ-VAS score and CCQ total score was moderate (r = -0.524 and r = -0.374; both p < 0.001). In conclusion, both generic and respiratory-specific quality of life are affected in non-hospitalized patients with COVID-19, approximately three months after the onset of symptoms. The combined use of the EQ-5D and the CCQ could identify the broad impact of COVID-19 on quality of life.
Keywords: COVID-19; Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2); quality of life.
Conflict of interest statement
F.M.E.F reports grants and personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GlaxoSmithKline, grants and personal fees from Novartis, personal fees from TEVA, outside the submitted work. D.J.A.J. reports personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Novartis, outside the submitted work. MAS reports grants from Lung Foundation Netherlands, grants from Stichting Astma Bestrijding, grants and personal fees from Boehringer Ingelheim, and grants and personal fees from AstraZeneca, outside the submitted work. R.M., J.M.D., Y.M.J.G., A.W.V., F.V.C.M., M.V.H., C.B., R.P., B.S., Y.S., H.V., A.V.H. and S.H.W. declare no conflict of interest.
Figures

References
-
- Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., De Ridder D., Petrovic D., Schrempft S., Marcus K., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. - DOI - PMC - PubMed
-
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F., et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. - DOI - PMC - PubMed
-
- Goërtz Y.M.J., Van Herck M., Delbressine J.M., Vaes A.W., Meys R., Machado F.V.C., Houben-Wilke S., Burtin C., Posthuma R., Franssen F.M.E., et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020;6 doi: 10.1183/23120541.00542-2020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

