Identification and quantitation of hinge cysteinylation on endogenous IgG2 antibodies from human serum
- PMID: 33317401
- PMCID: PMC7755172
- DOI: 10.1080/19420862.2020.1854923
Identification and quantitation of hinge cysteinylation on endogenous IgG2 antibodies from human serum
Abstract
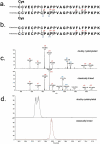
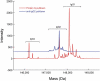
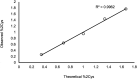
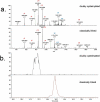
Cysteinylation is a post-translational modification (PTM) that occurs when a cysteine residue on a protein forms a disulfide bond with a terminal cysteine molecule. This PTM has been found in the hinge region of several recombinant therapeutic IgG2 antibodies, but the impact of cysteinylation on the safety and immunogenicity of therapeutics remains unclear. In this study, we characterized recombinant and endogenous IgG2 antibodies to quantify their levels of hinge cysteinylation, if present. To the best of our knowledge, this is the first study to identify and quantify hinge cysteinylation in endogenous IgG2 antibodies from healthy human serum. We used anti-IgG2 immunopurification of human serum to specifically enrich for endogenous IgG2 antibodies, and then subjected the resulting samples to Lys-C peptide mapping coupled with targeted mass spectrometry techniques. Using this analytical workflow, we found that all healthy human serum samples tested (N = 10) contained quantifiable levels of hinge cysteinylation (0.8 ± 0.3%) in their endogenous human IgG2s (IgG2-A isoform). These findings demonstrate that hinge cysteinylation in therapeutic IgG2s, at least up to a certain level, is well tolerated in humans and pose minimal safety or immunogenicity risks.
Keywords: IgG2; cysteinylation; disulfide; endogenous antibodies; hinge region; human serum.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
