Human breast milk as source of sphingolipids for newborns: comparison with infant formulas and commercial cow's milk
- PMID: 33317546
- PMCID: PMC7734711
- DOI: 10.1186/s12967-020-02641-0
Human breast milk as source of sphingolipids for newborns: comparison with infant formulas and commercial cow's milk
Abstract
Background: In the past two decades, sphingolipids have become increasingly appreciated as bioactive molecules playing important roles in a wide array of pathophysiology mechanisms. Despite advances in the field, sphingolipids as nutrients remain little explored. Today the research is starting to move towards the study of the sphingomyelin content in human breast milk, recommended for feeding infants.
Methods: In the present study, we performed a lipidomic analysis in human breast milk in relation with maternal diet during pregnancy, in infant formulas, and in commercial whole and semi-skimmed milks for adults. Mediterranean, carnivorous and vegetarian diets were considered.
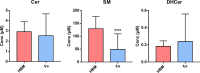
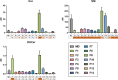
Results: The results showed that total sphingomyelin, ceramide and dihydroceramide species are independent on the diet. Interestingly, the milk sphingolipid composition is species-specific. In fact, infant formulas and commercial milks for adults have a lower level of total sphingomyelin and ceramide content than human breast milk with very different composition of each sphingolipid species.
Conclusions: We conclude that human breast milk is a better source of sphingolipids than infant formulas for baby nutrition with potential implications for the brain development and cognitive functions.
Keywords: Ceramide; Cow’s milk; Human breast milk; Infant formulas; Lipidomic; Sphingomyelin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

