Cost-effectiveness of the Adaptive Implementation of Effective Programs Trial (ADEPT): approaches to adopting implementation strategies
- PMID: 33317593
- PMCID: PMC7734829
- DOI: 10.1186/s13012-020-01069-w
Cost-effectiveness of the Adaptive Implementation of Effective Programs Trial (ADEPT): approaches to adopting implementation strategies
Abstract
Background: Theory-based methods to support the uptake of evidence-based practices (EBPs) are critical to improving mental health outcomes. Implementation strategy costs can be substantial, and few have been rigorously evaluated. The purpose of this study is to conduct a cost-effectiveness analysis to identify the most cost-effective approach to deploying implementation strategies to enhance the uptake of Life Goals, a mental health EBP.
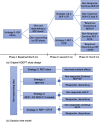
Methods: We used data from a previously conducted randomized trial to compare the cost-effectiveness of Replicating Effective Programs (REP) combined with external and/or internal facilitation among sites non-responsive to REP. REP is a low-level strategy that includes EBP packaging, training, and technical assistance. External facilitation (EF) involves external expert support, and internal facilitation (IF) augments EF with protected time for internal staff to support EBP implementation. We developed a decision tree to assess 1-year costs and outcomes for four implementation strategies: (1) REP only, (2) REP+EF, (3) REP+EF add IF if needed, (4) REP+EF/IF. The analysis used a 1-year time horizon and assumed a health payer perspective. Our outcome was quality-adjusted life years (QALYs). The economic outcome was the incremental cost-effectiveness ratio (ICER). We conducted deterministic and probabilistic sensitivity analysis (PSA).
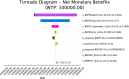
Results: Our results indicate that REP+EF add IF is the most cost-effective option with an ICER of $593/QALY. The REP+EF/IF and REP+EF only conditions are dominated (i.e., more expensive and less effective than comparators). One-way sensitivity analyses indicate that results are sensitive to utilities for REP+EF and REP+EF add IF. The PSA results indicate that REP+EF, add IF is the optimal strategy in 30% of iterations at the threshold of $100,000/QALY.
Conclusions: Our results suggest that the most cost-effective implementation support begins with a less intensive, less costly strategy initially and increases as needed to enhance EBP uptake. Using this approach, implementation support resources can be judiciously allocated to those clinics that would most benefit. Our results were not robust to changes in the utility measure. Research is needed that incorporates robust and relevant utilities in implementation studies to determine the most cost-effective strategies. This study advances economic evaluation of implementation by assessing costs and utilities across multiple implementation strategy combinations.
Trial registration: ClinicalTrials.gov Identifier: NCT02151331 , 05/30/2014.
Keywords: Cost-effectiveness analysis; Costs and cost analysis; Implementation science; Mental health services, community.
Conflict of interest statement
None.
Figures


References
-
- Kessler R, Heeringa S, Lakoma M, Petukhova M, Rupp AE, Schoenbaum M, et al. Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2008;165(6):703–711. doi: 10.1176/appi.ajp.2008.08010126. - DOI - PMC - PubMed
-
- Kilbourne A, Goodrich D, Nord K, Van Poppelen C, Kyle J, Bauer M, et al. Long-term clinical outcomes from a randomized controlled trial of two implementation strategies to promote collaborative care attendance in community practices. Adm Policy Ment Health. 2015;42(5):642–653. doi: 10.1007/s10488-014-0598-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

