Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses
- PMID: 33317695
- PMCID: PMC7744099
- DOI: 10.7554/eLife.57950
Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses
Abstract
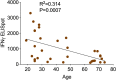
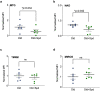
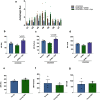
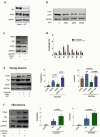
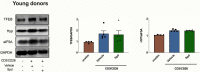
Vaccines are powerful tools to develop immune memory to infectious diseases and prevent excess mortality. In older adults, however vaccines are generally less efficacious and the molecular mechanisms that underpin this remain largely unknown. Autophagy, a process known to prevent aging, is critical for the maintenance of immune memory in mice. Here, we show that autophagy is specifically induced in vaccine-induced antigen-specific CD8+ T cells in healthy human volunteers. In addition, reduced IFNγ secretion by RSV-induced T cells in older vaccinees correlates with low autophagy levels. We demonstrate that levels of the endogenous autophagy-inducing metabolite spermidine fall in human T cells with age. Spermidine supplementation in T cells from old donors recovers their autophagy level and function, similar to young donors' cells, in which spermidine biosynthesis has been inhibited. Finally, our data show that endogenous spermidine maintains autophagy via the translation factor eIF5A and transcription factor TFEB. In summary, we have provided evidence for the importance of autophagy in vaccine immunogenicity in older humans and uncovered two novel drug targets that may increase vaccination efficiency in the aging context.
Trial registration: ClinicalTrials.gov NCT01070407 NCT01296451.
Keywords: TFEB; autophagy; human; human T cells; immunology; inflammation; spermidine; vaccine.
© 2020, Alsaleh et al.
Conflict of interest statement
GA, IP, LS, HZ, FR, AM, JL, EB, PK, CG, AS No competing interests declared
Figures







References
-
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O'Hara G, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. Novel Adenovirus-Based vaccines induce broad and sustained T cell responses to HCV in man. Science Translational Medicine. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

