Screening and Identification of Metacaspase Inhibitors: Evaluation of Inhibition Mechanism and Trypanocidal Activity
- PMID: 33318019
- PMCID: PMC8092552
- DOI: 10.1128/AAC.01330-20
Screening and Identification of Metacaspase Inhibitors: Evaluation of Inhibition Mechanism and Trypanocidal Activity
Abstract
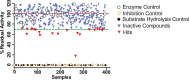
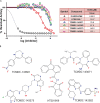
A common strategy to identify new antiparasitic agents is the targeting of proteases, due to their essential contributions to parasite growth and development. Metacaspases (MCAs) are cysteine proteases present in fungi, protozoa, and plants. These enzymes, which are associated with crucial cellular events in trypanosomes, are absent in the human host, thus arising as attractive drug targets. To find new MCA inhibitors with trypanocidal activity, we adapted a continuous fluorescence enzymatic assay to a medium-throughput format and carried out screening of different compound collections, followed by the construction of dose-response curves for the most promising hits. We used MCA5 from Trypanosoma brucei (TbMCA5) as a model for the identification of inhibitors from the GlaxoSmithKline HAT and CHAGAS chemical boxes. We also assessed a third collection of nine compounds from the Maybridge database that had been identified by virtual screening as potential inhibitors of the cysteine peptidase falcipain-2 (clan CA) from Plasmodium falciparum Compound HTS01959 (from the Maybridge collection) was the most potent inhibitor, with a 50% inhibitory concentration (IC50) of 14.39 µM; it also inhibited other MCAs from T. brucei and Trypanosoma cruzi (TbMCA2, 4.14 µM; TbMCA3, 5.04 µM; TcMCA5, 151 µM). HTS01959 behaved as a reversible, slow-binding, and noncompetitive inhibitor of TbMCA2, with a mechanism of action that included redox components. Importantly, HTS01959 displayed trypanocidal activity against bloodstream forms of T. brucei and trypomastigote forms of T. cruzi, without cytotoxic effects on Vero cells. Thus, HTS01959 is a promising starting point to develop more specific and potent chemical structures to target MCAs.
Keywords: Chagas disease; antiparasitic agent; inhibitors; metacaspases; sleeping sickness; target-based screening.
Copyright © 2021 American Society for Microbiology.
Figures






References
-
- Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM. 2000. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967. doi:10.1016/S1097-2765(00)00094-0. - DOI - PubMed
-
- Minina EA, Staal J, Alvarez VE, Berges JA, Berman-Frank I, Beyaert R, Bidle KD, Bornancin F, Casanova M, Cazzulo JJ, Choi CJ, Coll NS, Dixit VM, Dolinar M, Fasel N, Funk C, Gallois P, Gevaert K, Gutierrez-Beltran E, Hailfinger S, Klemenčič M, Koonin EV, Krappmann D, Linusson A, Machado MFM, Madeo F, Megeney LA, Moschou PN, Mottram JC, Nyström T, Osiewacz HD, Overall CM, Pandey KC, Ruland J, Salvesen GS, Shi Y, Smertenko A, Stael S, Ståhlberg J, Suárez MF, Thome M, Tuominen H, Van Breusegem F, van der Hoorn RAL, Vardi A, Zhivotovsky B, Lam E, Bozhkov PV. 2020. Classification and nomenclature of metacaspases and paracaspases: no more confusion with caspases. Mol Cell 77:927–929. doi:10.1016/j.molcel.2019.12.020. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

