Actin-ring segment switching drives nonadhesive gap closure
- PMID: 33318201
- PMCID: PMC7777027
- DOI: 10.1073/pnas.2010960117
Actin-ring segment switching drives nonadhesive gap closure
Abstract
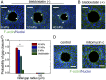
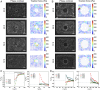
Gap closure to eliminate physical discontinuities and restore tissue integrity is a fundamental process in normal development and repair of damaged tissues and organs. Here, we demonstrate a nonadhesive gap closure model in which collective cell migration, large-scale actin-network fusion, and purse-string contraction orchestrate to restore the gap. Proliferative pressure drives migrating cells to attach onto the gap front at which a pluricellular actin ring is already assembled. An actin-ring segment switching process then occurs by fusion of actin fibers from the newly attached cells into the actin cable and defusion from the previously lined cells, thereby narrowing the gap. Such actin-cable segment switching occurs favorably at high curvature edges of the gap, yielding size-dependent gap closure. Cellular force microscopies evidence that a persistent rise in the radial component of inward traction force signifies successful actin-cable segment switching. A kinetic model that integrates cell proliferation, actin fiber fusion, and purse-string contraction is formulated to quantitatively account for the gap-closure dynamics. Our data reveal a previously unexplored mechanism in which cells exploit multifaceted strategies in a highly cooperative manner to close nonadhesive gaps.
Keywords: actin ring; cell patterning; gap closure; traction force microscopy.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Martin P., Wound healing–Aiming for perfect skin regeneration. Science 276, 75–81 (1997). - PubMed
-
- Jacinto A., et al. , Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr. Biol. 10, 1420–1426 (2000). - PubMed
-
- Wood W., et al. , Wound healing recapitulates morphogenesis in Drosophila embryos. Nat. Cell Biol. 4, 907–912 (2002). - PubMed
-
- Bement W. M., Mandato C. A., Kirsch M. N., Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr. Biol. 9, 579–587 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

