Hydroxyethylstarch revisited for acute brain injury treatment
- PMID: 33318420
- PMCID: PMC8284304
- DOI: 10.4103/1673-5374.300978
Hydroxyethylstarch revisited for acute brain injury treatment
Abstract
Infusion of the colloid hydroxyethylstarch has been used for volume substitution to maintain hemodynamics and microcirculation after e.g., severe blood loss. In the last decade it was revealed that hydroxyethylstarch can aggravate acute kidney injury, especially in septic patients. Because of the serious risk for critically ill patients, the administration of hydroxyethylstarch was restricted for clinical use. Animal studies and recently published in vitro experiments showed that hydroxyethylstarch might exert protective effects on the blood-brain barrier. Since the prevention of blood-brain barrier disruption was shown to go along with the reduction of brain damage after several kinds of insults, we revisit the topic hydroxyethylstarch and discuss a possible niche for the application of hydroxyethylstarch in acute brain injury treatment.
Keywords: acute subarachnoid hemorrhage; astrocyte; chronic kidney disease; delayed cerebral ischemia; microglia; neurovascular unit; osmotic pressure; pericyte; stroke; traumatic brain injury.
Conflict of interest statement
None
Figures
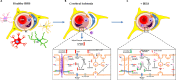
References
-
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69:777–784. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

