Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report
- PMID: 33318491
- PMCID: PMC7736571
- DOI: 10.1038/s41467-020-19761-2
Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report
Abstract
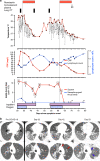
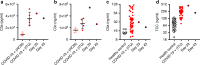
The response to the coronavirus disease 2019 (COVID-19) pandemic has been hampered by lack of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antiviral therapy. Here we report the use of remdesivir in a patient with COVID-19 and the prototypic genetic antibody deficiency X-linked agammaglobulinaemia (XLA). Despite evidence of complement activation and a robust T cell response, the patient developed persistent SARS-CoV-2 pneumonitis, without progressing to multi-organ involvement. This unusual clinical course is consistent with a contribution of antibodies to both viral clearance and progression to severe disease. In the absence of these confounders, we take an experimental medicine approach to examine the in vivo utility of remdesivir. Over two independent courses of treatment, we observe a temporally correlated clinical and virological response, leading to clinical resolution and viral clearance, with no evidence of acquired drug resistance. We therefore provide evidence for the antiviral efficacy of remdesivir in vivo, and its potential benefit in selected patients.
Conflict of interest statement
E.J.M.T. is an employee of Hycult Biotechnology Ltd. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- RG95376/MRC_/Medical Research Council/United Kingdom
- MR/S035362/1/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- MR/M501621/1/MRC_/Medical Research Council/United Kingdom
- 206298/B/17/Z/WT_/Wellcome Trust/United Kingdom
- MR/J014370/1/MRC_/Medical Research Council/United Kingdom
- 109965/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- WT109965MA/WT_/Wellcome Trust/United Kingdom
- MR/V011561/1/MRC_/Medical Research Council/United Kingdom
- 200871/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 207498/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- MR/V028448/1/MRC_/Medical Research Council/United Kingdom
- 210688/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MR/P008801/1/MRC_/Medical Research Council/United Kingdom
- MR/L006197/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00025/12/MRC_/Medical Research Council/United Kingdom
- HF-19-1595/HCRW_/HCRW_/United Kingdom
- MC_EX_MR/S300011/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

