Serum metabolites as biomarkers in systemic sclerosis-associated interstitial lung disease
- PMID: 33318574
- PMCID: PMC7736572
- DOI: 10.1038/s41598-020-78951-6
Serum metabolites as biomarkers in systemic sclerosis-associated interstitial lung disease
Abstract
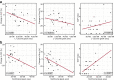
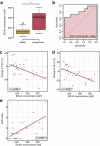
Systemic sclerosis (SSc) is a severe multi-organ disease with interstitial lung disease (ILD) being the major cause of death. While targeted therapies are emerging, biomarkers for sub-stratifying patients based on individual profiles are lacking. Herein, we investigated how levels of serum metabolites correlated with different stages of SSc and SSc-ILD. Serum samples of patients with SSc without ILD, stable and progressive SSc-ILD as well as of healthy controls (HC) were analysed using liquid targeted tandem mass spectrometry. The best discriminating profile consisted of 4 amino acids (AA) and 3 purine metabolites. L-tyrosine, L-tryptophan, and 1-methyl-adenosine distinguished HC from SSc patients. L-leucine, L-isoleucine, xanthosine, and adenosine monophosphate differentiated between progressing and stable SSc-ILD. In SSc-ILD, both, L-leucine and xanthosine negatively correlated with changes in FVC% predicted. Additionally, xanthosine was negatively correlated with changes in DLco% predicted and positively with the prognostic GAP index. Validation of L-leucine and L-isoleucine by an enzymatic assay confirmed both the sub-stratification of SSc-ILD patients and correlation with lung function and prognosis score. Serum metabolites may have potential as biomarkers for discriminating SSc patients based on the presence and severity of ILD. Confirmation in larger cohorts will be needed to appreciate their value for routine clinical care.
Conflict of interest statement
CM, KF, CB, JS, CN: none to declare. OD had speaker fees from Actelion, Bayer, Boehringer Ingelheim, Medscape, Novartis, Roche, Menarini, Mepha, MSD, iQone, Pfizer, consultancy fees from Abbvie, Actelion, Acceleron Pharma, Amgen, AnaMar, Bayer, Baecon Discovery, Blade Therapeutics, Boehringer, CSL Behring, ChemomAb, Curzion Pharmaceuticals, Ergonex, Galapagos NV, GSK, Glenmark Pharmaceuticals, Inventiva, Italfarmaco, iQvia, medac, Medscape, Mitsubishi Tanabe Pharma, MSD, Roche, Sanofi, UCB, Amgen, Lilly, Target BioScience, Pfizer, project scoring fees from Abbvie, interview fees from Catenion, travel support from Pfizer, research grants from Actelion, Bayer, Boehringer Ingelheim, Competitive Drug Development International Ltd, Mitsubishi Tanabe, patent issued: mir-29 for the treatment of systemic sclerosis (US8247389, EP2331143). BM had grant/research support from AbbVie, Protagen, Novartis, and congress support from Pfizer, Roche, Actelion, and MSD, speakers honorary from Novartis. In addition, B. Maurer has a patent mir-29 for the treatment of systemic sclerosis registered (US8247389, EP2331143). The real or perceived potential conflicts listed above are accurately stated. YA had consultancy relationships and/or has received research funding from Actelion, Bayer, Boehringer Ingelheim, ChemomAb, Genentech/Roche, Inventiva, Pfizer, Sanofi, Servier, in the area of potential treatments of scleroderma and its complications. AH received consulting fees and/or research funding from Actelion, ARXX, Bayer, Boehringer Ingelheim, Medscape, MSD and Roche.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

