Growth, ionic homeostasis, and physiological responses of cotton under different salt and alkali stresses
- PMID: 33318587
- PMCID: PMC7736318
- DOI: 10.1038/s41598-020-79045-z
Growth, ionic homeostasis, and physiological responses of cotton under different salt and alkali stresses
Abstract
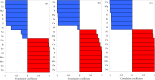
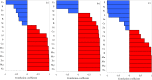
To better understand the mechanism of salt tolerance, we analyzed cotton growth and the ionomes in different tissues under different types of salt-alkali stress. Cotton was exposed to the soil salt and alkali stresses, NaCl, Na2SO4, and Na2CO3 + NaHCO3, in a pot study. Salt and alkali stress significantly inhibited cotton growth, significantly reduced root length, surface area, and volume, and significantly increased relative electrical conductivity (REC) and malondialdehyde (MDA) content but also significantly increased antioxidant enzyme activities, and proline (Pro) content. The REC in leaves was higher under salt stress than under alkali stress, but the effects on Pro were in the order Na2CO3 + NaHCO3 > NaCl > Na2SO4. Principal component analysis showed a significant difference in ion composition under the different types of salt-alkali stress. Under the three types of salt-alkali stress, concentrations of Na and Mo increased significantly in different organs of cotton plants. Under NaCl stress, the absorption of Ca was inhibited, the transport capacity of P, Mg, and Cu was reduced, and the ion balance was maintained by promoting the uptake and transport of Zn, Mn, Al, and Mo. Under Na2SO4 stress, the absorption of P and Ca was inhibited, the transport capacity of Mg, B, and Cu was reduced, and the ion balance was maintained by promoting the uptake and transport of S, Zn, Fe, Mo, Al, and Co. Under Na2CO3 + NaHCO3 stress, the absorption of P and S was inhibited, the transport capacity of Mg and B was reduced, but that of Al and Fe increased, and the ion balance was maintained by promoting the uptake and transport of Mn, Mo, Ni, and Co. The relative expression of GhSOS1 and GhNHX1 in leaves increased significantly under salt stress but decreased under alkali stress. These results suggest that cotton is well-adapted to salt-alkali stress via the antioxidant enzyme system, adjustment of osmotic substances, and reconstruction of ionic equilibrium; neutral salt stress primarily disrupts the ion balance, whereas alkali stress decreases the ability to regulate Na and inhibits the absorption of mineral elements, as well as disrupts the ion balance; and the changes in the expression of salt tolerance-related genes may partially explain the accumulation of Na ions in cotton under salt-alkali stress.
Conflict of interest statement
The authors declare no competing interests.
Figures












References
-
- Liu J, Guo WQ, Shi DC. Seed germination, seedling survival, and physiological response of sunflowers under saline and alkaline conditions. Photosynthetica. 2010;48(2):278–286. doi: 10.1007/s11099-010-0034-3. - DOI
-
- Cheng C, et al. Co-expression of AtNHX1 and TsVP improves the salt tolerance of transgenic cotton and increases seed cotton yield in a saline field. Mol. Breed. 2018;38(2):19–33. doi: 10.1007/s11032-018-0774-5. - DOI
-
- Shi L, Ma S, Fang Y, Xu J. Crucial variations in growth and ion homeostasis of Glycine gracilis seedlings under two types of salt stresses. J. Soil Sci. Plant Nutr. 2015;15(4):1007–1023.
Publication types
LinkOut - more resources
Full Text Sources

