Structures of radial spokes and associated complexes important for ciliary motility
- PMID: 33318703
- PMCID: PMC7855293
- DOI: 10.1038/s41594-020-00530-0
Structures of radial spokes and associated complexes important for ciliary motility
Abstract
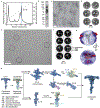
In motile cilia, a mechanoregulatory network is responsible for converting the action of thousands of dynein motors bound to doublet microtubules into a single propulsive waveform. Here, we use two complementary cryo-EM strategies to determine structures of the major mechanoregulators that bind ciliary doublet microtubules in Chlamydomonas reinhardtii. We determine structures of isolated radial spoke RS1 and the microtubule-bound RS1, RS2 and the nexin-dynein regulatory complex (N-DRC). From these structures, we identify and build atomic models for 30 proteins, including 23 radial-spoke subunits. We reveal how mechanoregulatory complexes dock to doublet microtubules with regular 96-nm periodicity and communicate with one another. Additionally, we observe a direct and dynamically coupled association between RS2 and the dynein motor inner dynein arm subform c (IDAc), providing a molecular basis for the control of motor activity by mechanical signals. These structures advance our understanding of the role of mechanoregulation in defining the ciliary waveform.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures














References
-
- Ginger ML, Portman N & McKean PG Swimming with protists: perception, motility and flagellum assembly. Nat Rev Micro 6, 838–850 (2008). - PubMed
-
- Lyons RA, Saridogan E & Djahanbakhch O The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update 12, 363–372 (2006). - PubMed
-
- Faubel R, Westendorf C, Bodenschatz E & Eichele G Cilia-based flow network in the brain ventricles. Science 353, 176–178 (2016). - PubMed
-
- Nonaka S et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

