Visualizing cellular heterogeneity by quantifying the dynamics of MAPK activity in live mammalian cells with synthetic fluorescent biosensors
- PMID: 33319088
- PMCID: PMC7723811
- DOI: 10.1016/j.heliyon.2020.e05574
Visualizing cellular heterogeneity by quantifying the dynamics of MAPK activity in live mammalian cells with synthetic fluorescent biosensors
Abstract
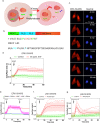
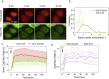
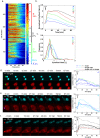
Mitogen-Activated Protein Kinases (MAPKs) control a wide array of cellular functions by transducing extracellular information into defined biological responses. In order to understand how these pathways are regulated, dynamic single cell measurements are highly needed. Fluorescence microscopy is well suited to perform these measurements. However, more dynamic and sensitive biosensors that allow the quantification of signaling activity in living mammalian cells are required. We have engineered a synthetic fluorescent substrate for human MAPKs (ERK, JNK and p38) that relocates from the nucleus to the cytoplasm when phosphorylated by the kinases. We demonstrate that this reporter displays an improved response compared to other relocation biosensors. This assay allows to monitor the heterogeneity in the MAPK response in a population of isogenic cells, revealing pulses of ERK activity upon a physiological EGFR stimulation. We show applicability of this approach to the analysis of multiple cancer cell lines and primary cells as well as its application in vivo to developing tumors. Using this ERK biosensor, dynamic single cell measurements with high temporal resolution can be obtained. These MAPK reporters can be widely applied to the analysis of molecular mechanisms of MAPK signaling in healthy and diseased state, in cell culture assays or in vivo.
Keywords: Biochemistry; Cancer research; Cell biology; Fluorescent biosensor; Live-cell imaging; MAPK signaling; Single cells; Systems biology.
© 2020 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

