Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis
- PMID: 33319187
- PMCID: PMC7724188
- DOI: 10.1016/j.jhepr.2020.100191
Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis
Abstract
Background & aims: Biochemical markers, including GLOBE score and aspartate aminotransferase-to-platelet ratio index (APRI), are used to stratify risk in patients with primary biliary cholangitis (PBC). This study aimed to evaluate the effects of obeticholic acid (OCA) on categorical shifts in GLOBE score, APRI, and both combined, based on data from POISE, a phase III placebo-controlled trial in patients with PBC who had an incomplete response or were intolerant to ursodeoxycholic acid.
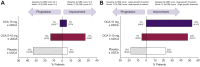
Methods: In a post hoc analysis, baseline and Month 12 data from POISE were used to calculate the APRI and GLOBE score. Patients were stratified into 3 risk groups based on a combination of APRI (0.54) and GLOBE (0.3 or age-specific) thresholds.
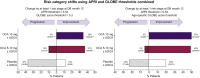
Results: The analysis included 215 patients (47 low risk; 79 moderate risk; 89 high risk). Using the combined GLOBE score (threshold of 0.3) and APRI thresholds, there was improvement in ≥1 risk stage in 37% and 35% of patients in the OCA 5-10 mg and 10 mg groups, respectively, vs. 12% in the placebo group (both p <0.05). Progression occurred in 10% and 0% in the 5-10 mg and 10 mg groups vs. 37% in the placebo group. Results with GLOBE age-specific thresholds were similar.
Conclusions: Based on change in APRI and GLOBE score at 12 months, OCA treatment is associated with reduction in the predicted risk of liver-related complications in patients with PBC.
Lay summary: Primary biliary cholangitis (PBC) is a chronic disease affecting the liver. People who suffer from PBC are at risk of serious long-term complications. Information from certain blood tests can be used to estimate the likelihood of experiencing long-term complications. The results of this study showed that based on blood test results, people taking obeticholic acid, with or without ursodeoxycholic acid, for PBC were predicted to have a better outcome than those taking placebo.
Clinical trials registration: NCT01473524.
Keywords: AE, adverse event; ALP, alkaline phosphatase; APRI; APRI, aspartate aminotransferase-to-platelet ratio index; AST, aspartate aminotransferase; Cholestasis; DB, double-blind; FXR, farnesoid X receptor; IQR, inter-quartile range; LLN, lower limit of normal; LN, natural logarithm; LT, liver transplant; OCA, obeticholic acid; OR, odds ratio; PBC; PBC, primary biliary cholangitis; Risk stratification; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
© 2020 Published by Elsevier B.V. on behalf of European Association for the Study of the Liver (EASL).
Conflict of interest statement
Maren H. Harms has received speaker fees from Zambon BV. Gideon M. Hirschfield has received consultancy fees from CymaBay, Gilead, GSK, Intercept, and Novartis, as well as grant funding from Gilead and Falk Pharma. Annarosa Floreani declares no conflicts of interest that pertain to this work. Marlyn J. Mayo has served on advisory committees or review panels for GSK, and has received grant/research support from Gilead, CymaBay, Intercept, Mallinckrodt, Novartis, Target, GSK, and Genfit. Albert Parés has received grant funding, personal fees, and advisory board fees from Intercept; personal fees and advisory board fee from Novartis; and personal fees from CymaBay and Inova Diagnostics. Alexander Liberman and Leigh MacConell are employees and shareholders of Intercept. Elizabeth Smoot Malecha is an employee of Intercept. Richard Pencek is a shareholder and former employee of Intercept. Bettina E. Hansen has received grant funding and personal fees from Intercept, CymaBay, and Albireo, and has received personal fees from Mirum and ChemoMab. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. - PubMed
-
- Lindor K.D., Bowlus C.L., Boyer J., Levy C., Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. - PubMed
-
- Harms M.H., Lammers W.J., Thorburn D., Corpechot C., Invernizzi P., Janssen H.L.A. Major hepatic complications in ursodeoxycholic acid-treated patients with primary biliary cholangitis: risk factors and time trends in incidence and outcome. Am J Gastroenterol. 2018;113:254–264. - PubMed
-
- D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. - PubMed
-
- Harms M.H., van Buuren H.R., Corpechot C., Thorburn D., Janssen H.L.A., Lindor K.D. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71:357–365. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

