Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination
- PMID: 33319249
- PMCID: PMC9071282
- DOI: 10.1093/infdis/jiaa761
Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination
Abstract
Background: Takeda's dengue vaccine is under evaluation in an ongoing phase 3 efficacy study; we present a 2-year update.
Methods: Children (20 099, 4-16 years old) were randomized to receive 2 doses of TAK-003 or placebo 3 months apart and are under surveillance to detect dengue by serotype-specific RT-PCR.
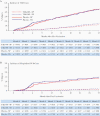
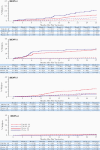
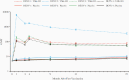
Results: Cumulative efficacy against dengue approximately 27 months since first dose was 72.7% (95% confidence interval [CI], 67.1%-77.3%), including 67.0% (95% CI, 53.6%-76.5%) in dengue-naive and 89.2% (95% CI, 82.4%-93.3%) against hospitalized dengue. In the second year, decline in efficacy was observed (56.2%; 95% CI, 42.3%-66.8%) with the largest decline in 4-5 year olds (24.5%; 95% CI, -34.2% to 57.5%); efficacy was 60.6% (95% CI, 43.8%-72.4%) in 6-11 year and 71.2% (95% CI, 41.0%-85.9%) in 12-16 year age groups. As TAK-003 efficacy varies by serotype, changes in serotype dominance partially contributed to efficacy differences in year-by-year analysis. No related serious adverse events occurred during the second year.
Conclusions: TAK-003 demonstrated continued benefit independent of baseline serostatus in reducing dengue with some decline in efficacy during the second year. Three-year data will be important to see if efficacy stabilizes or declines further.Clinical Trials Registration. NCT02747927.Takeda's tetravalent dengue vaccine (TAK-003) continued to demonstrate benefit in reducing dengue independent of baseline serostatus up to 2 years after completing vaccination with some decline in efficacy during the second year in 4-16 year olds in dengue-endemic countries.
Keywords: TAK-003; dengue; efficacy; immunogenicity; persistence; safety; vaccine.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- World Health Organization. Dengue vaccine: WHO position paper—September 2018. Wkly Epidemiol Rec 2018; 93:457–76.
-
- Sridhar S, Luedtke A, Langevin E, et al. . Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018; 379:327–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

