Primary and promiscuous functions coexist during evolutionary innovation through whole protein domain acquisitions
- PMID: 33319743
- PMCID: PMC7790495
- DOI: 10.7554/eLife.58061
Primary and promiscuous functions coexist during evolutionary innovation through whole protein domain acquisitions
Abstract
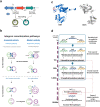
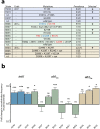
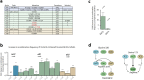
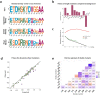
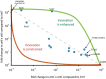
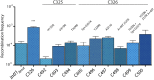
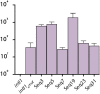
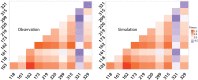
Molecular examples of evolutionary innovation are scarce and generally involve point mutations. Innovation can occur through larger rearrangements, but here experimental data is extremely limited. Integron integrases innovated from double-strand- toward single-strand-DNA recombination through the acquisition of the I2 α-helix. To investigate how this transition was possible, we have evolved integrase IntI1 to what should correspond to an early innovation state by selecting for its ancestral activity. Using synonymous alleles to enlarge sequence space exploration, we have retrieved 13 mutations affecting both I2 and the multimerization domains of IntI1. We circumvented epistasis constraints among them using a combinatorial library that revealed their individual and collective fitness effects. We obtained up to 104-fold increases in ancestral activity with various asymmetrical trade-offs in single-strand-DNA recombination. We show that high levels of primary and promiscuous functions could have initially coexisted following I2 acquisition, paving the way for a gradual evolution toward innovation.
Keywords: E. coli; domain acquisition; evolution; evolutionary biology; innovation; integrase; integron; recombination.
© 2020, Escudero et al.
Conflict of interest statement
JE, AN, HK, CL, OT, DM No competing interests declared
Figures







References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CNRS-UMR3525/Centre National de la Recherche Scientifique/International
- BIO2017-85056-P/Ministerio de Ciencia, Innovacion y Universidades de Espana/International
- FDT20150532465/Fondation pour la Recherche Médicale/International
- PIEF-GA-2011-303022/EU-MSC Actions/International
- 282004/EU FP7 HEALTH/International
- 612146/EU-FP7 FET/International
- DBF20160635736/Fondation pour la Recherche Médicale/International
- ANR-10-LABX-62-IBEID/Agence Nationale de la Recherche/International
- ANR-12- 897 BLAN-DynamINT/Agence Nationale de la Recherche/International
- 2016-T1/BIO-1105/Comunidad de Madrid/International
- PIEF-GA-2011-303022/H2020 Marie Skłodowska-Curie Actions/International
- StG-803375/ERC_/European Research Council/International
- BIO2017-85056-P/Ministerio de Ciencia e Innovación/International
- EQU201903007848/Fondation pour la Recherche Médicale/International
- 310944/European Union 7th Framework Programme/International
- FP-7-PEOPLE2011-IEF/Pierre and Marie Curie University/International
- ICADIGE/Pierre and Marie Curie University/International
LinkOut - more resources
Full Text Sources

