Alterations in resting-state network dynamics along the Alzheimer's disease continuum
- PMID: 33319785
- PMCID: PMC7738511
- DOI: 10.1038/s41598-020-76201-3
Alterations in resting-state network dynamics along the Alzheimer's disease continuum
Abstract
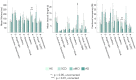
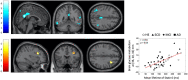
Human brain activity is intrinsically organized into resting-state networks (RSNs) that transiently activate or deactivate at the sub-second timescale. Few neuroimaging studies have addressed how Alzheimer's disease (AD) affects these fast temporal brain dynamics, and how they relate to the cognitive, structural and metabolic abnormalities characterizing AD. We aimed at closing this gap by investigating both brain structure and function using magnetoencephalography (MEG) and hybrid positron emission tomography-magnetic resonance (PET/MR) in 10 healthy elders, 10 patients with subjective cognitive decline (SCD), 10 patients with amnestic mild cognitive impairment (aMCI) and 10 patients with typical Alzheimer's disease with dementia (AD). The fast activation/deactivation state dynamics of RSNs were assessed using hidden Markov modeling (HMM) of power envelope fluctuations at rest measured with MEG. Correlations were sought between temporal properties of HMM states and participants' cognitive test scores, whole hippocampal grey matter volume and regional brain glucose metabolism. The posterior default-mode network (DMN) was less often activated and for shorter durations in AD patients than matched healthy elders. No significant difference was found in patients with SCD or aMCI. The time spent by participants in the activated posterior DMN state did not correlate significantly with cognitive scores, nor with the whole hippocampal volume. However, it correlated positively with the regional glucose consumption in the right dorsolateral prefrontal cortex (DLPFC). AD patients present alterations of posterior DMN power activation dynamics at rest that identify an additional electrophysiological correlate of AD-related synaptic and neural dysfunction. The right DLPFC may play a causal role in the activation of the posterior DMN, possibly linked to the occurrence of mind wandering episodes. As such, these data might suggest a neural correlate of the decrease in mind wandering episodes reported in pathological aging.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Thal, D. R., Rüb, U., Orantes, M. & Braak, H. Phases of ANL-deposition in the human brain and its relevance for the development of AD. 11 (2002). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

