Clinical utility of genomic sequencing: a measurement toolkit
- PMID: 33319814
- PMCID: PMC7738524
- DOI: 10.1038/s41525-020-00164-7
Clinical utility of genomic sequencing: a measurement toolkit
Abstract
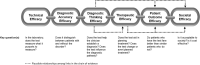
Whole-genome sequencing (WGS) is positioned to become one of the most robust strategies for achieving timely diagnosis of rare genomic diseases. Despite its favorable diagnostic performance compared to conventional testing strategies, routine use and reimbursement of WGS are hampered by inconsistencies in the definition and measurement of clinical utility. For example, what constitutes clinical utility for WGS varies by stakeholder's perspective (physicians, patients, families, insurance companies, health-care organizations, and society), clinical context (prenatal, pediatric, critical care, adult medicine), and test purpose (diagnosis, screening, treatment selection). A rapidly evolving technology landscape and challenges associated with robust comparative study design in the context of rare disease further impede progress in this area of empiric research. To address this challenge, an expert working group of the Medical Genome Initiative was formed. Following a consensus-based process, we align with a broad definition of clinical utility and propose a conceptually-grounded and empirically-guided measurement toolkit focused on four domains of utility: diagnostic thinking efficacy, therapeutic efficacy, patient outcome efficacy, and societal efficacy. For each domain of utility, we offer specific indicators and measurement strategies. While we focus on diagnostic applications of WGS for rare germline diseases, this toolkit offers a flexible framework for best practices around measuring clinical utility for a range of WGS applications. While we expect this toolkit to evolve over time, it provides a resource for laboratories, clinicians, and researchers looking to characterize the value of WGS beyond the laboratory.
Conflict of interest statement
John Belmont and Stacie Taylor are employed by and shareholders of Illumina Inc. Robert Green receives compensation for advising AIA, SavvySherpa, Verily, Wamberg; and is co-founder of Genome Medical. All other authors declare no competing interests.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

