Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation
- PMID: 33320179
- PMCID: PMC7958144
- DOI: 10.1210/endrev/bnaa032
Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation
Abstract
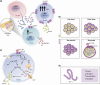
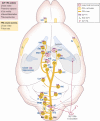
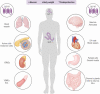
Glucagon-like peptide-1 (GLP-1) is produced in gut endocrine cells and in the brain, and acts through hormonal and neural pathways to regulate islet function, satiety, and gut motility, supporting development of GLP-1 receptor (GLP-1R) agonists for the treatment of diabetes and obesity. Classic notions of GLP-1 acting as a meal-stimulated hormone from the distal gut are challenged by data supporting production of GLP-1 in the endocrine pancreas, and by the importance of brain-derived GLP-1 in the control of neural activity. Moreover, attribution of direct vs indirect actions of GLP-1 is difficult, as many tissue and cellular targets of GLP-1 action do not exhibit robust or detectable GLP-1R expression. Furthermore, reliable detection of the GLP-1R is technically challenging, highly method dependent, and subject to misinterpretation. Here we revisit the actions of GLP-1, scrutinizing key concepts supporting gut vs extra-intestinal GLP-1 synthesis and secretion. We discuss new insights refining cellular localization of GLP-1R expression and integrate recent data to refine our understanding of how and where GLP-1 acts to control inflammation, cardiovascular function, islet hormone secretion, gastric emptying, appetite, and body weight. These findings update our knowledge of cell types and mechanisms linking endogenous vs pharmacological GLP-1 action to activation of the canonical GLP-1R, and the control of metabolic activity in multiple organs.
Keywords: brain; cardiovascular; diabetes; gastrointestinal tract; islets; obesity; receptor.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Comment in
-
GLP-1 receptor - Do we really know what we're looking at?Acta Histochem. 2021 Jul;123(5):151732. doi: 10.1016/j.acthis.2021.151732. Epub 2021 May 18. Acta Histochem. 2021. PMID: 34015646 No abstract available.
References
-
- Drucker DJ. Evolving concepts and translational relevance of enteroendocrine cell biology. J Clin Endocrinol Metab. 2016;101(3):778-786. - PubMed
-
- Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304(5924):368-371. - PubMed
-
- Heinrich G, Gros P, Lund PK, Bentley RC, Habener JF. Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology. 1984;115(6):2176-2181. - PubMed
-
- Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95(2):513-548. - PubMed

