Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery
- PMID: 33320196
- PMCID: PMC8168826
- DOI: 10.1093/neuonc/noaa278
Cell-surface antigen profiling of pediatric brain tumors: B7-H3 is consistently expressed and can be targeted via local or systemic CAR T-cell delivery
Abstract
Background: Immunotherapy with chimeric antigen receptor (CAR) T cells is actively being explored for pediatric brain tumors in preclinical models and early phase clinical studies. At present, it is unclear which CAR target antigens are consistently expressed across different pediatric brain tumor types. In addition, the extent of HLA class I expression is unknown, which is critical for tumor recognition by conventional αβTCR T cells.
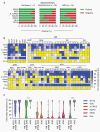
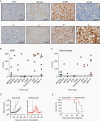
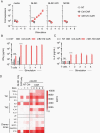
Methods: We profiled 49 low- and high-grade pediatric brain tumor patient-derived orthotopic xenografts (PDOX) by flow analysis for the expression of 5 CAR targets (B7-H3, GD2, IL-13Rα2, EphA2, and HER2), and HLA class I. In addition, we generated B7-H3-CAR T cells and evaluated their antitumor activity in vitro and in vivo.
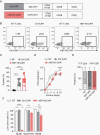
Results: We established an expression hierarchy for the analyzed antigens (B7-H3 = GD2 >> IL-13Rα2 > HER2 = EphA2) and demonstrated that antigen expression is heterogenous. All high-grade gliomas expressed HLA class I, but only 57.1% of other tumor subtypes had detectable expression. We then selected B7-H3 as a target for CAR T-cell therapy. B7-H3-CAR T cells recognized tumor cells in an antigen-dependent fashion. Local or systemic administration of B7-H3-CAR T cells induced tumor regression in PDOX and immunocompetent murine glioma models resulting in a significant survival advantage.
Conclusions: Our study highlights the importance of studying target antigen and HLA class I expression in PDOX samples for the future design of immunotherapies. In addition, our results support active preclinical and clinical exploration of B7-H3-targeted CAR T-cell therapies for a broad spectrum of pediatric brain tumors.
Keywords: B7-H3; CAR T-cell immunotherapy; GD2; HLA; immunocompetent and PDOX models; pediatric brain tumor.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Are B7-H3 CAR-T cells the future universal treatment for pediatric brain tumors?Neuro Oncol. 2021 Jun 1;23(6):872-873. doi: 10.1093/neuonc/noab063. Neuro Oncol. 2021. PMID: 33755151 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

