Comparison of Rates of Overdose and Hospitalization After Initiation of Medication for Opioid Use Disorder in the Inpatient vs Outpatient Setting
- PMID: 33320266
- PMCID: PMC7739119
- DOI: 10.1001/jamanetworkopen.2020.29676
Comparison of Rates of Overdose and Hospitalization After Initiation of Medication for Opioid Use Disorder in the Inpatient vs Outpatient Setting
Abstract
Importance: Whereas outpatient treatment with medication for opioid use disorder (MOUD) is evidence based, there is a large network of inpatient facilities in the US that are reimbursed by commercial insurers and do not typically offer MOUD.
Objective: To compare the rates of opioid-related overdose and all-cause hospitalization after outpatient MOUD treatment vs inpatient care.
Design, setting, and participants: This comparative effectiveness research study used deidentified claims of commercially insured individuals in the US from the MarketScan Commercial Claims and Encounters Database from January 1, 2010, to December 31, 2017, to obtain a sample of 37 090 individuals with opioid use disorder who initiated treatment with inpatient care and/or MOUD. Data were analyzed from October 1, 2019, to May 1, 2020. To address nonrandom treatment assignment, individuals with opioid use disorder who initiated MOUD or who entered inpatient care were matched 1:1 based on propensity scores.
Exposures: The independent variable of interest was the type of treatment initiated. Individuals could initiate 1 of 5 potential treatments: (1) outpatient MOUD, (2) short-term inpatient care, (3) short-term inpatient care followed by outpatient MOUD within 30 days, (4) long-term inpatient care, or (5) long-term inpatient care followed by outpatient MOUD within 30 days.
Main outcomes and measures: Opioid-related overdose and all-cause hospitalization at any point within the 12 months after treatment of opioid use disorder. The hazard for each outcome was estimated using a time-to-event Cox proportional hazards regression model.
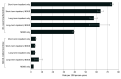
Results: The cohort included 37 090 individuals matched 1:1 between inpatient and outpatient treatment (20 723 [56%] were younger than 30 years; 23 250 [63%] were male). After propensity score matching, compared with the inpatient treatments, initiation of outpatient MOUD alone was followed by the lowest 1-year overdose rate (2.2 [95% CI, 2.0-2.5] per 100 person-years vs 3.5 [95% CI, 2.7-4.4] to 7.0 [95% CI, 4.6-10.7] per 100 person-years) and hospitalization rate (39 [95% CI, 38-40] per 100 person-years vs 57 [95% CI, 54-61] to 74 [95% CI, 73-76] per 100 person-years). Outpatient MOUD was also associated with the lowest hazard of these events compared with inpatient care, which had hazard ratios ranging from 1.71 (95% CI, 1.35-2.17) to 2.67 (95% CI, 1.68-4.23) for overdose and 1.33 (95% CI, 1.23-1.44) to 1.90 (95% CI, 1.83-1.97) for hospitalizations.
Conclusions and relevance: The results of this comparative effectiveness research study suggest that lower rates of subsequent overdose and hospitalization are associated with outpatient MOUD compared with short- or long-term inpatient care. When patients and clinicians have a choice of treatment, outpatient MOUD treatment may be associated with lower overdose and hospitalization on balance. Future research should assess which patients benefit most from inpatient care and how best to leverage existing inpatient treatment infrastructure.
Conflict of interest statement
Figures
References
-
- Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90-96. doi:10.1016/j.jsat.2017.07.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical