Gastrodin prevents homocysteine-induced human umbilical vein endothelial cells injury via PI3K/Akt/eNOS and Nrf2/ARE pathway
- PMID: 33320446
- PMCID: PMC7810955
- DOI: 10.1111/jcmm.16073
Gastrodin prevents homocysteine-induced human umbilical vein endothelial cells injury via PI3K/Akt/eNOS and Nrf2/ARE pathway
Abstract
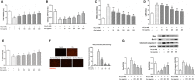
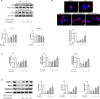
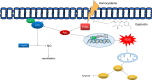
In this study, we investigated the protective effects of gastrodin (Gas) against homocysteine-induced human umbilical vein endothelial cell (HUVEC) injury and the role of the phosphoinositide 3-kinase (PI3K)/threonine kinase 1 (Akt)/endothelial nitric oxide synthase (eNOS) and NF-E2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathways. We stimulated cells with homocysteine (1 mmol/L, 24 hours) and tested the effects of gastrodin (200-800 μg/mL) on cell viability and the production of malondialdehyde (MDA), lactate dehydrogenase (LDH) and reactive oxygen species (ROS). Then, Nrf2 distribution in the cytoplasm and nucleus as well as the expression of enzymes downstream of Nrf2 was determined. Furthermore, we analysed the expression of bax, bcl-2 and cleaved caspase3, and assessed the involvement of the PI3K/Akt/eNOS pathway by Western blots. Finally, we tested the vasoactive effect of gastrodin in thoracic aortic rings. The results showed that gastrodin decreased MDA, LDH and ROS production and increased cell viability, NO production and relaxation of thoracic aortic rings. Moreover, the protective effects of Gas on NO production and relaxation of thoracic aortic rings were blocked by L-NAME but enhanced by Cav-1 knockdown, and MK-2206 treatment abolished the effect of Gas on the ROS. In addition, treatment with gastrodin increased Nrf2 nuclear translocation, thus enhancing the expression of downstream enzymes. Finally, gastrodin increased the expression of PI3K, p-Akt, and eNOS and decreased Cav-1 protein expression. In conclusion, our study suggested that gastrodin may protect HUVECs from homocysteine-induced injury, and the PI3K/Akt/eNOS and Nrf2/ARE pathways may be responsible for the efficacy of gastrodin.
Keywords: Cav-1/eNOS; Gastrodin; Nrf2; homocysteine; oxidative stress.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare there is no conflicts of interest regarding the publication of this paper.
Figures


References
-
- Mansoor MA, Bergmark C, Haswell SJ, et al. Correlation between plasma total homocysteine and copper in patients with peripheral vascular disease. Clin Chem. 2000;46:385‐391. - PubMed
-
- Mercie P, Garnier O, Lascoste L, et al. Homocysteine‐thiolactone induces caspase‐independent vascular endothelial cell death with apoptotic features. Apoptosis. 2000;5:403‐411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

