Clinical, laboratory, and temporal predictors of neutralizing antibodies against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates
- PMID: 33320842
- PMCID: PMC7843229
- DOI: 10.1172/JCI144930
Clinical, laboratory, and temporal predictors of neutralizing antibodies against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates
Abstract
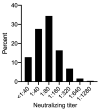
BACKGROUNDSARS-CoV-2-specific antibodies may protect from reinfection and disease, providing rationale for administration of plasma containing SARS-CoV-2-neutralizing antibodies (nAbs) as a treatment for COVID-19. Clinical factors and laboratory assays to streamline plasma donor selection, and the durability of nAb responses, are incompletely understood.METHODSPotential convalescent plasma donors with virologically documented SARS-CoV-2 infection were tested for serum IgG against SARS-CoV-2 spike protein S1 domain and against nucleoprotein (NP), and for nAb.RESULTSAmong 250 consecutive persons, including 27 (11%) requiring hospitalization, who were studied a median of 67 days since symptom onset, 97% were seropositive on 1 or more assays. Sixty percent of donors had nAb titers ≥1:80. Correlates of higher nAb titers included older age (adjusted OR [AOR] 1.03 per year of age, 95% CI 1.00-1.06), male sex (AOR 2.08, 95% CI 1.13-3.82), fever during illness (AOR 2.73, 95% CI 1.25-5.97), and disease severity represented by hospitalization (AOR 6.59, 95% CI 1.32-32.96). Receiver operating characteristic analyses of anti-S1 and anti-NP antibody results yielded cutoffs that corresponded well with nAb titers, with the anti-S1 assay being slightly more predictive. nAb titers declined in 37 of 41 paired specimens collected a median of 98 days (range 77-120) apart (P < 0.001). Seven individuals (2.8%) were persistently seronegative and lacked T cell responses.CONCLUSIONnAb titers correlated with COVID-19 severity, age, and sex. SARS-CoV-2 IgG results can serve as useful surrogates for nAb testing. Functional nAb levels declined, and a small proportion of convalescent individuals lacked adaptive immune responses.FUNDINGThe project was supported by the Frederick National Laboratory for Cancer Research with support from the NIAID under contract number 75N91019D00024, and was supported by the Fred Hutchinson Joel Meyers Endowment, Fast-Grants, a New Investigator award from the American Society for Transplantation and Cellular Therapy, and NIH contracts 75N93019C0063, 75N91019D00024, and HHSN272201800013C, and NIH grants T32-AI118690, T32-AI007044, K08-AI119142, and K23-AI140918.
Keywords: Adaptive immunity; Immunoglobulins; Infectious disease; Virology.
Conflict of interest statement
Figures



Update of
-
Clinical, laboratory, and temporal predictors of neutralizing antibodies to SARS-CoV-2 after COVID-19.medRxiv [Preprint]. 2020 Oct 21:2020.10.06.20207472. doi: 10.1101/2020.10.06.20207472. medRxiv. 2020. Update in: J Clin Invest. 2021 Feb 1;131(3):144930. doi: 10.1172/JCI144930. PMID: 33052361 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

