Exact neural mass model for synaptic-based working memory
- PMID: 33320855
- PMCID: PMC7771880
- DOI: 10.1371/journal.pcbi.1008533
Exact neural mass model for synaptic-based working memory
Abstract
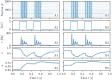
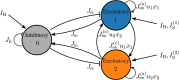
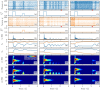
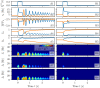
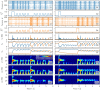
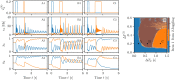
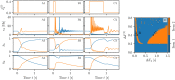
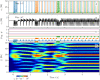
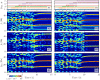
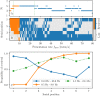
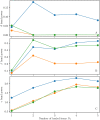
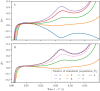
A synaptic theory of Working Memory (WM) has been developed in the last decade as a possible alternative to the persistent spiking paradigm. In this context, we have developed a neural mass model able to reproduce exactly the dynamics of heterogeneous spiking neural networks encompassing realistic cellular mechanisms for short-term synaptic plasticity. This population model reproduces the macroscopic dynamics of the network in terms of the firing rate and the mean membrane potential. The latter quantity allows us to gain insight of the Local Field Potential and electroencephalographic signals measured during WM tasks to characterize the brain activity. More specifically synaptic facilitation and depression integrate each other to efficiently mimic WM operations via either synaptic reactivation or persistent activity. Memory access and loading are related to stimulus-locked transient oscillations followed by a steady-state activity in the β-γ band, thus resembling what is observed in the cortex during vibrotactile stimuli in humans and object recognition in monkeys. Memory juggling and competition emerge already by loading only two items. However more items can be stored in WM by considering neural architectures composed of multiple excitatory populations and a common inhibitory pool. Memory capacity depends strongly on the presentation rate of the items and it maximizes for an optimal frequency range. In particular we provide an analytic expression for the maximal memory capacity. Furthermore, the mean membrane potential turns out to be a suitable proxy to measure the memory load, analogously to event driven potentials in experiments on humans. Finally we show that the γ power increases with the number of loaded items, as reported in many experiments, while θ and β power reveal non monotonic behaviours. In particular, β and γ rhythms are crucially sustained by the inhibitory activity, while the θ rhythm is controlled by excitatory synapses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A Spiking Working Memory Model Based on Hebbian Short-Term Potentiation.J Neurosci. 2017 Jan 4;37(1):83-96. doi: 10.1523/JNEUROSCI.1989-16.2016. J Neurosci. 2017. PMID: 28053032 Free PMC article.
-
Modelling the formation of working memory with networks of integrate-and-fire neurons connected by plastic synapses.J Physiol Paris. 2003 Jul-Nov;97(4-6):659-81. doi: 10.1016/j.jphysparis.2004.01.021. J Physiol Paris. 2003. PMID: 15242673 Review.
-
Theta and gamma power increases and alpha/beta power decreases with memory load in an attractor network model.J Cogn Neurosci. 2011 Oct;23(10):3008-20. doi: 10.1162/jocn_a_00029. Epub 2011 Mar 31. J Cogn Neurosci. 2011. PMID: 21452933
-
Cholinergic modulation of cortical oscillatory dynamics.J Neurophysiol. 1995 Jul;74(1):288-97. doi: 10.1152/jn.1995.74.1.288. J Neurophysiol. 1995. PMID: 7472331
-
Neural mechanisms of attending to items in working memory.Neurosci Biobehav Rev. 2019 Jun;101:1-12. doi: 10.1016/j.neubiorev.2019.03.017. Epub 2019 Mar 26. Neurosci Biobehav Rev. 2019. PMID: 30922977 Free PMC article. Review.
Cited by
-
Neuronal Cascades Shape Whole-Brain Functional Dynamics at Rest.eNeuro. 2021 Oct 28;8(5):ENEURO.0283-21.2021. doi: 10.1523/ENEURO.0283-21.2021. Print 2021 Sep-Oct. eNeuro. 2021. PMID: 34583933 Free PMC article.
-
Mean-Field Approximations With Adaptive Coupling for Networks With Spike-Timing-Dependent Plasticity.Neural Comput. 2023 Aug 7;35(9):1481-1528. doi: 10.1162/neco_a_01601. Neural Comput. 2023. PMID: 37437202 Free PMC article.
-
Neurophysiological avenues to better conceptualizing adaptive cognition.Commun Biol. 2024 May 24;7(1):626. doi: 10.1038/s42003-024-06331-1. Commun Biol. 2024. PMID: 38789522 Free PMC article.
-
Whole brain functional connectivity: Insights from next generation neural mass modelling incorporating electrical synapses.PLoS Comput Biol. 2024 Dec 5;20(12):e1012647. doi: 10.1371/journal.pcbi.1012647. eCollection 2024 Dec. PLoS Comput Biol. 2024. PMID: 39637233 Free PMC article.
-
Robust and brain-like working memory through short-term synaptic plasticity.PLoS Comput Biol. 2022 Dec 27;18(12):e1010776. doi: 10.1371/journal.pcbi.1010776. eCollection 2022 Dec. PLoS Comput Biol. 2022. PMID: 36574424 Free PMC article.
References
-
- Fuster JM. Memory in the cerebral cortex: An empirical approach to neural networks in the human and nonhuman primate. MIT press; 1999.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

