Targeting complement components C3 and C5 for the retina: Key concepts and lingering questions
- PMID: 33321207
- PMCID: PMC8197769
- DOI: 10.1016/j.preteyeres.2020.100936
Targeting complement components C3 and C5 for the retina: Key concepts and lingering questions
Abstract
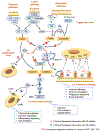
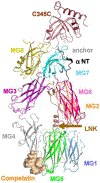
Age-related macular degeneration (AMD) remains a major cause of legal blindness, and treatment for the geographic atrophy form of AMD is a significant unmet need. Dysregulation of the complement cascade is thought to be instrumental for AMD pathophysiology. In particular, C3 and C5 are pivotal components of the complement cascade and have become leading therapeutic targets for AMD. In this article, we discuss C3 and C5 in detail, including their roles in AMD, biochemical and structural aspects, locations of expression, and the functions of C3 and C5 fragments. Further, the article critically reviews developing therapeutics aimed at C3 and C5, underscoring the potential effects of broad inhibition of complement at the level of C3 versus more specific inhibition at C5. The relationships of complement biology to the inflammasome and microglia/macrophage activity are highlighted. Concepts of C3 and C5 biology will be emphasized, while we point out questions that need to be settled and directions for future investigations.
Keywords: Age-related macular degeneration; C3; C5; Complement; Geographic atrophy; Retina.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
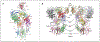
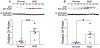


References
-
- Alcorlo M, Martínez-Barricarte R, Fernández FJ, Rodríguez-Gallego C, Round A, Vega MC, Harris CL, Rodríguez De Cordoba S, Llorca O, 2011. Unique structure of iC3b resolved at a resolution of 24 Å by 3D-electron microscopy. Proc. Natl. Acad. Sci. U. S. A 108, 13236–13240. 10.1073/pnas.1106746108. - DOI - PMC - PubMed
-
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Bowes Rickman C, Bok D, Hageman GS, Johnson LV, 2010. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res 29 (2), 95–112. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

