High Mobility Group Box-1 and Blood-Brain Barrier Disruption
- PMID: 33321691
- PMCID: PMC7764171
- DOI: 10.3390/cells9122650
High Mobility Group Box-1 and Blood-Brain Barrier Disruption
Abstract
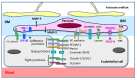
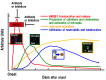
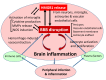
Increasing evidence suggests that inflammatory responses are involved in the progression of brain injuries induced by a diverse range of insults, including ischemia, hemorrhage, trauma, epilepsy, and degenerative diseases. During the processes of inflammation, disruption of the blood-brain barrier (BBB) may play a critical role in the enhancement of inflammatory responses and may initiate brain damage because the BBB constitutes an interface between the brain parenchyma and the bloodstream containing blood cells and plasma. The BBB has a distinct structure compared with those in peripheral tissues: it is composed of vascular endothelial cells with tight junctions, numerous pericytes surrounding endothelial cells, astrocytic endfeet, and a basement membrane structure. Under physiological conditions, the BBB should function as an important element in the neurovascular unit (NVU). High mobility group box-1 (HMGB1), a nonhistone nuclear protein, is ubiquitously expressed in almost all kinds of cells. HMGB1 plays important roles in the maintenance of chromatin structure, the regulation of transcription activity, and DNA repair in nuclei. On the other hand, HMGB1 is considered to be a representative damage-associated molecular pattern (DAMP) because it is translocated and released extracellularly from different types of brain cells, including neurons and glia, contributing to the pathophysiology of many diseases in the central nervous system (CNS). The regulation of HMGB1 release or the neutralization of extracellular HMGB1 produces beneficial effects on brain injuries induced by ischemia, hemorrhage, trauma, epilepsy, and Alzheimer's amyloidpathy in animal models and is associated with improvement of the neurological symptoms. In the present review, we focus on the dynamics of HMGB1 translocation in different disease conditions in the CNS and discuss the functional roles of extracellular HMGB1 in BBB disruption and brain inflammation. There might be common as well as distinct inflammatory processes for each CNS disease. This review will provide novel insights toward an improved understanding of a common pathophysiological process of CNS diseases, namely, BBB disruption mediated by HMGB1. It is proposed that HMGB1 might be an excellent target for the treatment of CNS diseases with BBB disruption.
Keywords: blood–brain barrier; high mobility group box-1; inflammation; monoclonal antibody; pericyte; stroke; trauma; vascular endothelial cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

