Involvement of Bradykinin Receptor 2 in Nerve Growth Factor Neuroprotective Activity
- PMID: 33321704
- PMCID: PMC7763563
- DOI: 10.3390/cells9122651
Involvement of Bradykinin Receptor 2 in Nerve Growth Factor Neuroprotective Activity
Abstract
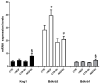
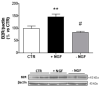
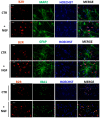
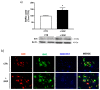
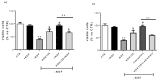
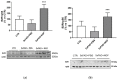
Neurotrophin nerve growth factor (NGF) has been demonstrated to upregulate the gene expression of bradykinin receptor 2 (B2R) on sensory neurons, thus facilitating nociceptive signals. The aim of the present study is to investigate the involvement of B2R in the NGF mechanism of action in nonsensory neurons in vitro by using rat mixed cortical primary cultures (CNs) and mouse hippocampal slices, and in vivo in Alzheimer's disease (AD) transgenic mice (5xFAD) chronically treated with NGF. A significant NGF-mediated upregulation of B2R was demonstrated by microarray, Western blot, and immunofluorescence analysis in CNs, indicating microglial cells as the target of this modulation. The B2R involvement in the NGF mechanism of action was also demonstrated by using a selective B2R antagonist which was able to reverse the neuroprotective effect of NGF in CNs, as revealed by viability assay, and the NGF-induced long-term potentiation (LTP) in hippocampal slices. To confirm in vitro observations, B2R upregulation was observed in 5xFAD mouse brain following chronic intranasal NGF treatment. This study demonstrates for the first time that B2R is a key element in the neuroprotective activity and synaptic plasticity mediated by NGF in brain cells.
Keywords: Alzheimer’s disease animal models; LTP; bradykinin receptor 2 (B2R); microglial cells; nerve growth factor (NGF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

