NO Scavenging through Reductive Nitrosylation of Ferric Mycobacterium tuberculosis and Homo sapiens Nitrobindins
- PMID: 33321752
- PMCID: PMC7763097
- DOI: 10.3390/ijms21249395
NO Scavenging through Reductive Nitrosylation of Ferric Mycobacterium tuberculosis and Homo sapiens Nitrobindins
Abstract
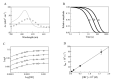
Ferric nitrobindins (Nbs) selectively bind NO and catalyze the conversion of peroxynitrite to nitrate. In this study, we show that NO scavenging occurs through the reductive nitrosylation of ferric Mycobacterium tuberculosis and Homo sapiens nitrobindins (Mt-Nb(III) and Hs-Nb(III), respectively). The conversion of Mt-Nb(III) and Hs-Nb(III) to Mt-Nb(II)-NO and Hs-Nb(II)-NO, respectively, is a monophasic process, suggesting that over the explored NO concentration range (between 2.5 × 10-5 and 1.0 × 10-3 M), NO binding is lost in the mixing time (i.e., NOkon ≥ 1.0 × 106 M-1 s-1). The pseudo-first-order rate constant for the reductive nitrosylation of Mt-Nb(III) and Hs-Nb(III) (i.e., k) is not linearly dependent on the NO concentration but tends to level off, with a rate-limiting step (i.e., klim) whose values increase linearly with [OH-]. This indicates that the conversion of Mt-Nb(III) and Hs-Nb(III) to Mt-Nb(II)-NO and Hs-Nb(II)-NO, respectively, is limited by the OH--based catalysis. From the dependence of klim on [OH-], the values of the second-order rate constant kOH- for the reductive nitrosylation of Mt-Nb(III)-NO and Hs-Nb(III)-NO were obtained (4.9 (±0.5) × 103 M-1 s-1 and 6.9 (±0.8) × 103 M-1 s-1, respectively). This process leads to the inactivation of two NO molecules: one being converted to HNO2 and another being tightly bound to the ferrous heme-Fe(II) atom.
Keywords: NO scavenging; heme-protein; nitrobindin; reductive nitrosylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ascenzi P., Brunori M. A molecule for all seasons: The heme. J. Porphyr. Phthalocyanines. 2016;20:134–149. doi: 10.1142/S1088424616300081. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

