Ionone Is More than a Violet's Fragrance: A Review
- PMID: 33321809
- PMCID: PMC7764282
- DOI: 10.3390/molecules25245822
Ionone Is More than a Violet's Fragrance: A Review
Abstract
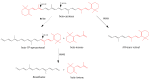
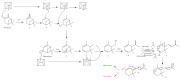
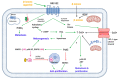
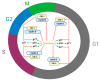
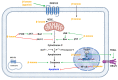
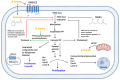
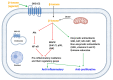
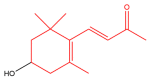
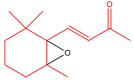
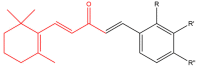
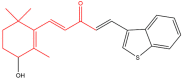
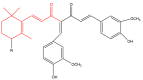
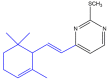
The term ionone is derived from "iona" (Greek for violet) which refers to the violet scent and "ketone" due to its structure. Ionones can either be chemically synthesized or endogenously produced via asymmetric cleavage of β-carotene by β-carotene oxygenase 2 (BCO2). We recently proposed a possible metabolic pathway for the conversion of α-and β-pinene into α-and β-ionone. The differences between BCO1 and BCO2 suggest a unique physiological role of BCO2; implying that β-ionone (one of BCO2 products) is involved in a prospective biological function. This review focuses on the effects of ionones and the postulated mechanisms or signaling cascades involved mediating these effects. β-Ionone, whether of an endogenous or exogenous origin possesses a range of pharmacological effects including anticancer, chemopreventive, cancer promoting, melanogenesis, anti-inflammatory and antimicrobial actions. β-Ionone mediates these effects via activation of olfactory receptor (OR51E2) and regulation of the activity or expression of cell cycle regulatory proteins, pro-apoptotic and anti-apoptotic proteins, HMG-CoA reductase and pro-inflammatory mediators. α-Ionone and β-ionone derivatives exhibit anti-inflammatory, antimicrobial and anticancer effects, however the corresponding structure activity relationships are still inconclusive. Overall, data demonstrates that ionone is a promising scaffold for cancer, inflammation and infectious disease research and thus is more than simply a violet's fragrance.
Keywords: BCO2; OR51E2; biological activity; cancer; inflammation; ionone; ionone derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

