Recent Strategies and Applications for l-Asparaginase Confinement
- PMID: 33321857
- PMCID: PMC7764279
- DOI: 10.3390/molecules25245827
Recent Strategies and Applications for l-Asparaginase Confinement
Abstract
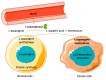
l-asparaginase (ASNase, EC 3.5.1.1) is an aminohydrolase enzyme with important uses in the therapeutic/pharmaceutical and food industries. Its main applications are as an anticancer drug, mostly for acute lymphoblastic leukaemia (ALL) treatment, and in acrylamide reduction when starch-rich foods are cooked at temperatures above 100 °C. Its use as a biosensor for asparagine in both industries has also been reported. However, there are certain challenges associated with ASNase applications. Depending on the ASNase source, the major challenges of its pharmaceutical application are the hypersensitivity reactions that it causes in ALL patients and its short half-life and fast plasma clearance in the blood system by native proteases. In addition, ASNase is generally unstable and it is a thermolabile enzyme, which also hinders its application in the food sector. These drawbacks have been overcome by the ASNase confinement in different (nano)materials through distinct techniques, such as physical adsorption, covalent attachment and entrapment. Overall, this review describes the most recent strategies reported for ASNase confinement in numerous (nano)materials, highlighting its improved properties, especially specificity, half-life enhancement and thermal and operational stability improvement, allowing its reuse, increased proteolysis resistance and immunogenicity elimination. The most recent applications of confined ASNase in nanomaterials are reviewed for the first time, simultaneously providing prospects in the described fields of application.
Keywords: acrylamide mitigation; biosensors; confinement strategies; l-asparaginase; nanomaterials; therapeutic agents.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Karpel-Massler G., Ramani D., Shu C., Halatsch M.-E., Westhoff M.-A., Bruce J.N., Canoll P., Siegelin M.D. Metabolic reprogramming of glioblastoma cells by l-asparaginase sensitizes for apoptosis in vitro and in vivo. Oncotarget. 2016;7:33512–33528. doi: 10.18632/oncotarget.9257. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

