Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort
- PMID: 33321879
- PMCID: PMC7764571
- DOI: 10.3390/brainsci10120962
Treatment-Resistant Depression in a Real-World Setting: First Interim Analysis of Characteristics, Healthcare Resource Use, and Utility Values of the FondaMental Cohort
Abstract
Background: Major depressive disorder (MDD) is among the most common psychiatric disorders. One-third of patients are usually unresponsive to several lines of treatment. This study aimed to describe the FondaMental French cohort of patients with treatment-resistant depression (TRD) and to estimate utility and healthcare resource use outcomes.
Methods: Patients with TRD were evaluated prospectively over four years (baseline, 6, 12, 18, 24, 36 and 48 months) in a real-world clinical setting. Interim analyses focused on the first two consecutive years. Four MDD-related states (major depressive episode (MDE), response, remission, recovery) were defined based on the MADRS (Montgomery-Åsberg depression rating scale) and other clinical events. Health status was assessed with the EuroQol 5 Dimensions 5 Level (EQ-5D-5L) questionnaire. Utility values were estimated as preference measures that the patients assigned to their overall health status.
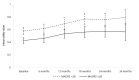
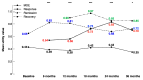
Results: This study was based on 252 patients with TRD. The mean utility value by health state was 0.41, 0.63, 0.80, and 0.90, for MDE, response, remission, and recovery, respectively. At baseline, 59% of patients had an MADRS score of at least 28. Their baseline average utility value was lower compared to the other patients (0.43 versus 0.58, p < 0.001). This significant difference persisted at the following visits. The rate of patients in MDEs having at least one hospitalisation for depression or other reasons than depression was generally higher than that in the other health states.
Conclusion: This study documented patterns in healthcare resource consumption, quality of life, and other characteristics in patients with TRD, both globally and by health state and depression severity.
Keywords: healthcare resource use; real-world; treatment-resistant depression; utility.
Conflict of interest statement
Antoine Yrondi: received speaker’s honoraria (AstraZeneca, Janssen, Lundbeck, Otsuka, Servier), and carried out clinical studies in relation to the development of a medicine (Janssen, Lundbeck) unrelated to this work. Olivier Doumy received honoraria from Lilly, Astra-Zeneca, Janssen, Servier, Lundbeck. Jean Baptiste Genty received speaker’s honoraria from Servier. Pierre Michel Llorca received grants, honoraria, and consulting fees from Allergan, Gedeon Richter, Janssen-Cilag, Lundbeck, Otsuka, Recordati, Sanofi-Aventis and Teva. Raphael Rachieri received speaker’s honoraria from Janssen Cilag. Ludovic Samalin received grants, honoraria, and consulting fees from Janssen-Cilag, Lundbeck, and Otsuka. Florian Stephan received honoraria from Otsuka. Emmanuel Haffen acted in advisory capacities, carried out clinical studies in relation to the development of a medicine, received personal research, studies or travel allowances, gave presentations at meetings, and received remuneration for input from the following pharmaceutical organisations: AstraZeneca, BMS, Cellgene, Euthérapie—Servier, Janssen, Elli Lilly, Lundbeck, LivaNova, Otsuka, Pfizer, Sanofi. They also held a managerial position in the FondaMental Foundation (Créteil) and the French Association of Biological Psychiatry and Neuropsychopharmacology. Wissam El-Hage reports receiving speaker’s honoraria from Chugai, Eisai, Lundbeck, Janssen-Cilag, Otsuka, and UCB unrelated to this work. Bruno Aouizerate received speaker’s honoraria and a travel allowance from Lundbeck, Janssen-Cilag, Sanofi and Eli Lilly. He has served on the advisory board of Janssen-Cilag. Etienne Allauze, Thierry d’Amato, Djamila Bennabi, Thierry Bougerol, Vincent Camus, Philippe Courtet, Jérôme Holtzmann, Christophe Lançon, Marion Leboyer, Julia Maruani, Rémi Moirand, Fanny Molière, Isabel Nieto, Damien Pierre, Michel Walter Jean Petrucci, Laurent Schmitt, Guillaume Vaiva, Sophie Marguet, and Natalie Dennis declare themselves to have no conflicts of interest.
Figures
References
-
- Chevance A., Gaillard R. La dépression, du mal-être à la maladie. Bull. Epidémiol Hebd. 2018;32:636–637.
-
- IsHak W.W., Mirocha J., James D., Tobia G., Vilhauer J., Fakhry H., Pi S., Hanson E., Nashawati R., Peselow E.D., et al. Quality of life in major depressive disorder before/after multiple steps of treatment and one-year follow-up. Acta Psychiatr. Scand. 2015;131:51–60. doi: 10.1111/acps.12301. - DOI - PMC - PubMed
-
- Nierenberg A.A., Husain M.M., Trivedi M.H., Fava M., Warden D., Wisniewski S.R., Miyahara S., Rush A.J. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: A STAR*D report. Psychol. Med. 2010;40:41–50. doi: 10.1017/S0033291709006011. - DOI - PMC - PubMed
-
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. - DOI - PubMed
-
- Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources