Recent Advances in the Synthesis of Selenophenes and Their Derivatives
- PMID: 33322179
- PMCID: PMC7764687
- DOI: 10.3390/molecules25245907
Recent Advances in the Synthesis of Selenophenes and Their Derivatives
Abstract
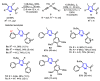
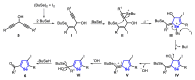
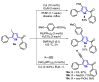
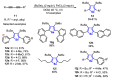
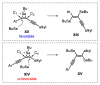
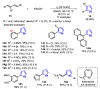
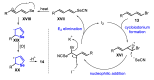
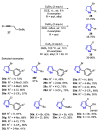
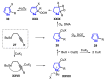
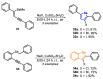
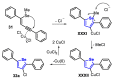
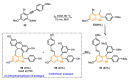
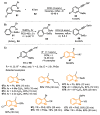
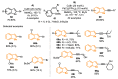
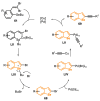
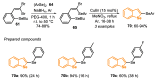
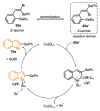
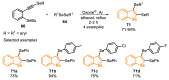
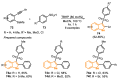
The selenophene derivatives are an important class of selenium-based heterocyclics. These compounds play an important role in prospecting new drugs, as well as in the development of new light-emitting materials. During the last years, several methods have been emerging to access the selenophene scaffold, employing a diversity of cyclization-based synthetic strategies, involving specific reaction partners and particularities. This review presents a comprehensive discussion on the recent advances in the synthesis of selenophene-based compounds, starting from different precursors, highlighting the main differences, the advantages, and limitations among them.
Keywords: benzoselenophene; cyclization; organochalcogen; organoselenium; selenophene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
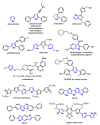

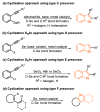
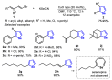
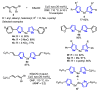
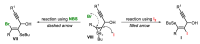




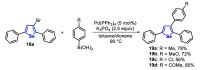
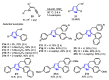
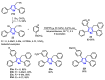

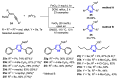




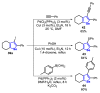
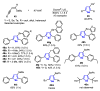
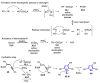
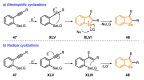





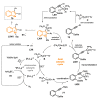

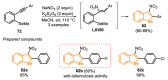
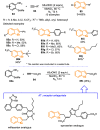
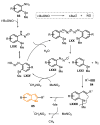





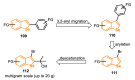

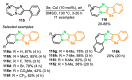
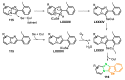

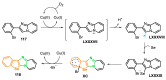



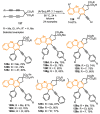
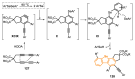
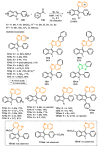

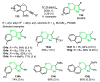

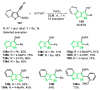
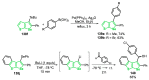
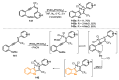

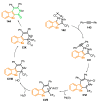
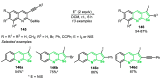
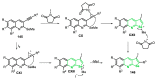

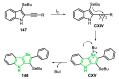
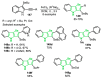
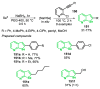
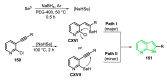
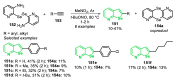
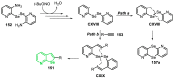
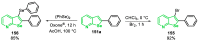


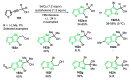
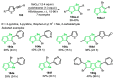
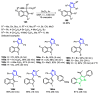
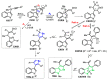


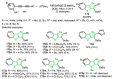
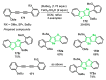

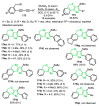


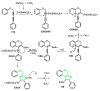
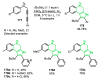
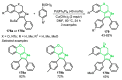

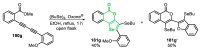
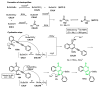
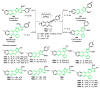
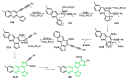
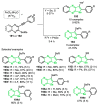
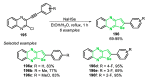

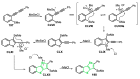
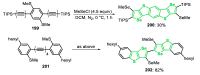
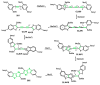
References
-
- Lenardão E.J., Santi C., Sancineto L. Organoselenium Compounds as Reagents and Catalysts to Develop New Green Protocols. Springer; Cham, Switzerland: 2018. pp. 1–98.
-
- Singh F.V., Wirth T. Selenium Reagents as Catalysts. Catal. Sci. Technol. 2019;9:1073–1091. doi: 10.1039/C8CY02274G. - DOI
-
- Shao L., Li Y., Lu J., Jiang X. Recent Progress in Selenium-catalyzed Organic Reactions. Org. Chem. Front. 2019;6:2999–3041. doi: 10.1039/C9QO00620F. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

