Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice
- PMID: 33322180
- PMCID: PMC7763936
- DOI: 10.3390/ijms21249483
Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice
Abstract
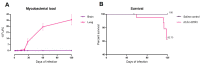
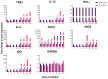
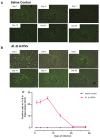
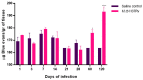
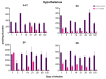
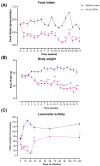
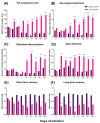
Tuberculosis (TB) is a chronic infectious disease in which prolonged, non-resolutive inflammation of the lung may lead to metabolic and neuroendocrine dysfunction. Previous studies have reported that individuals coursing pulmonary TB experience cognitive or behavioural changes; however, the pathogenic substrate of such manifestations have remained unknown. Here, using a mouse model of progressive pulmonary TB, we report that, even in the absence of brain infection, TB is associated with marked increased synthesis of both inflammatory and anti-inflammatory cytokines in discrete brain areas such as the hypothalamus, the hippocampal formation and cerebellum accompanied by substantial changes in the synthesis of neurotransmitters. Moreover, histopathological findings of neurodegeneration and neuronal death were found as infection progressed with activation of p38, JNK and reduction in the BDNF levels. Finally, we perform behavioural analysis in infected mice throughout the infection, and our data show that the cytokine and neurochemical changes were associated with a marked onset of cognitive impairment as well as depressive- and anxiety-like behaviour. Altogether, our results suggest that besides pulmonary damage, TB is accompanied by an extensive neuroinflammatory and neurodegenerative state which explains some of the behavioural abnormalities found in TB patients.
Keywords: Mycobacterium tuberculosis; behaviour abnormalities; neuroinflammation.
Conflict of interest statement
The authors of this manuscript declare that there are no actual or potential conflicts of interest. The authors affirm that there are no financial, personal or other relationships with other people or organisations that have inappropriately influenced or biased their research.
Figures










References
-
- World Health Organization . Global tuberculosis Report 2020. World Health Organization; Geneva, Switzerland: 2020. Licence: CC BY-NC-SA 3.0 IGO.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

