Retinoic Acid and Its Derivatives in Skin
- PMID: 33322246
- PMCID: PMC7764495
- DOI: 10.3390/cells9122660
Retinoic Acid and Its Derivatives in Skin
Abstract
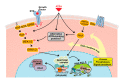
The retinoids are a group of compounds including vitamin A and its active metabolite all-trans-retinoic acid (ATRA). Retinoids regulate a variety of physiological functions in multiple organ systems, are essential for normal immune competence, and are involved in the regulation of cell growth and differentiation. Vitamin A derivatives have held promise in cancer treatment and ATRA is used in differentiation therapy of acute promyelocytic leukemia (APL). ATRA and other retinoids have also been successfully applied in a variety of dermatological conditions such as skin cancer, psoriasis, acne, and ichthyosis. Moreover, modulation of retinoic acid receptors and retinoid X (or rexinoid) receptors function may affect dermal cells. The studies using complex genetic models with various combinations of retinoic acid receptors (RARs) and retinoid X (or rexinoid) receptors (RXRs) indicate that retinoic acid and its derivatives have therapeutic potential for a variety of serious dermatological disorders including some malignant conditions. Here, we provide a synopsis of the main advances in understanding the role of ATRA and its receptors in dermatology.
Keywords: all-trans-retinoic acid; dermatology; retinoic acid receptors; vitamin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Khalil S., Bardawil T., Stephan C., Darwiche N., Abbas O., Kibbi A.G., Nemer G., Kurban M. Retinoids: A Journey from the Molecular Structures and Mechanisms of Action to Clinical Uses in Dermatology and Adverse Effects. J. Dermatol. Treat. 2017;28:684–696. doi: 10.1080/09546634.2017.1309349. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

