CuSO4/[Cu(NH3)4]SO4-Composite Thermochemical Energy Storage Materials
- PMID: 33322267
- PMCID: PMC7763518
- DOI: 10.3390/nano10122485
CuSO4/[Cu(NH3)4]SO4-Composite Thermochemical Energy Storage Materials
Abstract
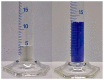
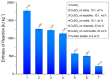
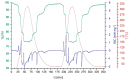
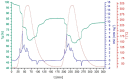
The thermochemical energy-storage material couple CuSO4/[Cu(NH3)4]SO4 combines full reversibility, application in a medium temperature interval (<350 °C), and fast liberation of stored heat. During reaction with ammonia, a large change in the sulfate solid-state structure occurs, resulting in a 2.6-fold expansion of the bulk material due to NH3 uptake. In order to limit this volume work, as well as enhance the thermal conductivity of the solid material, several composites of anhydrous CuSO4 with inorganic inert support materials were prepared and characterized with regard to their energy storage density, reversibility of the storage reaction, thermal conductivity, and particle morphology. The best thermochemical energy storage properties were obtained for a 10:1 CuSO4-sepiolite composite, combining an attractive energy storage density with slightly improved thermal conductivity and decreased bulk volume work compared to the pure salt.
Keywords: CuSO4/[Cu(NH3)4]SO4; composite material; thermal conductivity; thermochemical energy storage; thermochemistry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



References
-
- Shine K.P., Fuglestvedt J.S., Hailemariam K., Stuber N. Alternatives to the Global Warming Potential for Comparing Climate Impacts of Emissions of Greenhouse Gases. Clim. Chang. 2005;68:281–302. doi: 10.1007/s10584-005-1146-9. - DOI
-
- IEA . Heating without Global Warming: Market Developments and Policy Considerations for Renewable Heat. IEA; Paris, France: 2014.
-
- UN Treatie . Paris Agreement, No. 54113. UN Treatie; Geneva, Switzerland: 2015.
-
- IEA Co-Generation and Renewables. Solutions for a Low-Carbon Energy Future. [(accessed on 12 October 2020)];2011 Available online: https://www.iea.org/publications/freepublications/publication/co-generat....
-
- Abedin A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011;4:42–46. doi: 10.2174/1876387101004010042. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

