Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety
- PMID: 33322288
- PMCID: PMC7763646
- DOI: 10.3390/molecules25245852
Design, Synthesis and Fungicidal Activity of New 1,2,4-Triazole Derivatives Containing Oxime Ether and Phenoxyl Pyridinyl Moiety
Abstract
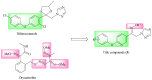
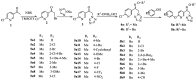
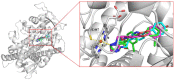
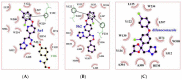
A series of novel 1,2,4-triazole derivatives containing oxime ether and phenoxy pyridine moiety were designed and synthesized. The new compounds were identified by nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS). Compound (Z)-1-(6-(4-nitrophenoxy)pyridin-3-yl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one O-methyl oxime (5a18) was further confirmed by X-ray single crystal diffraction. Their antifungal activities were evaluated against eight phytopathogens. The in vitro bioassays indicated that most of the title compounds displayed moderate to high fungicidal activities. Compound (Z)-1-(6-(4-bromo-2-chlorophenoxy)pyridin-3-yl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one O-methyl oxime (5a4) exhibited a broad-spectrum antifungal activities with the EC50 values of 1.59, 0.46, 0.27 and 11.39 mg/L against S. sclerotiorum, P. infestans, R. solani and B. cinerea, respectively. Compound (Z)-1-(6-(2-chlorophenoxy)pyridin-3-yl)-2-(1H-1,2,4-triazol-1-yl)ethan-1-one O-benzyl oxime (5b2) provided the lowest EC50 value of 0.12 mg/L against S. sclerotiorum, which were comparable to the commercialized difenoconazole. Moreover, homologous modeling and molecular docking disclosed possible binding modes of compounds 5a4 and 5b2 with CYP51. This work provided useful guidance for the discovery of new 1,2,4-triazole fungicides.
Keywords: 1,2,4-triazole; CYP51; fungicidal activity; oxime ether; phenoxy pyridinyl.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Oerke E.C., Dehne H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004;23:275–285. doi: 10.1016/j.cropro.2003.10.001. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

