A New Ammonium Smart Sensor with Interference Rejection
- PMID: 33322346
- PMCID: PMC7764669
- DOI: 10.3390/s20247102
A New Ammonium Smart Sensor with Interference Rejection
Abstract
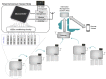
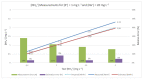
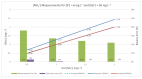
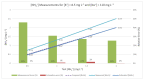
In many water samples, it is important to determine the ammonium concentration in order to obtain an overall picture of the environmental impact of pollutants and human actions, as well as to detect the stage of eutrophization. Ion selective electrodes (ISEs) have been commonly utilized for this purpose, although the presence of interfering ions (potassium and sodium in the case of NH4+-ISE) represents a handicap in terms of the measurement quality. Furthermore, random malfunctions may give rise to incorrect measurements. Bearing all of that in mind, a smart ammonium sensor with enhanced features has been developed and tested in water samples, as demonstrated and commented on in detail following the presentation of the complete set of experimental measurements that have been successfully carried out. This has been achieved through the implementation of an expert system that supervises a set of ISEs in order to (a) avoid random failures and (b) reject interferences. Our approach may also be suitable for in-line monitoring of the water quality through the implementation of wireless sensor networks.
Keywords: expert system; in-line water monitoring; interference tolerance; smart ammonium sensor; triple modular redundancy; wireless sensor networks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
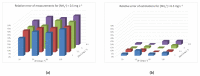
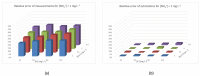
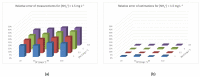
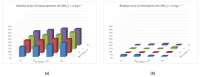
References
-
- Molins-Legua C., Meseguer-Lloret S., Moliner-Martinez Y., Campíns-Falcó P. A guide for selecting the most appropriate method for ammonium determination in water analysis. TrAC Trends Anal. Chem. 2006;25:282–290. doi: 10.1016/j.trac.2005.12.002. - DOI
-
- Nollet L.M., Gelder L.S., editors. Handbook of Water Analysis. 3rd ed. CRC Press; Boca Raton, FL, USA: 2013.
-
- Zhu Y., Chen J., Yuan D., Yang Z., Shi X., Li H., Jin H., Ran L. Development of analytical methods for ammonium determination in seawater over the last two decades. TrAC Trends Anal. Chem. 2019;119:115627. doi: 10.1016/j.trac.2019.115627. - DOI
-
- Liu J. New directions in sensor technology. TrAC Trends Anal. Chem. 2020;124:115818. doi: 10.1016/j.trac.2020.115818. - DOI
-
- Crespo G.A. Recent Advances in Ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta. 2017;245:1023–1034. doi: 10.1016/j.electacta.2017.05.159. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

