Pathology of Coronavirus Infections: A Review of Lesions in Animals in the One-Health Perspective
- PMID: 33322366
- PMCID: PMC7764021
- DOI: 10.3390/ani10122377
Pathology of Coronavirus Infections: A Review of Lesions in Animals in the One-Health Perspective
Abstract
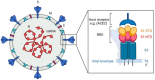
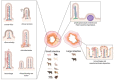
Coronaviruses (CoVs) are worldwide distributed RNA-viruses affecting several species, including humans, and causing a broad spectrum of diseases. Historically, they have not been considered a severe threat to public health until two outbreaks of COVs-related atypical human pneumonia derived from animal hosts appeared in 2002 and in 2012. The concern related to CoVs infection dramatically rose after the COVID-19 global outbreak, for which a spill-over from wild animals is also most likely. In light of this CoV zoonotic risk, and their ability to adapt to new species and dramatically spread, it appears pivotal to understand the pathophysiology and mechanisms of tissue injury of known CoVs within the "One-Health" concept. This review specifically describes all CoVs diseases in animals, schematically representing the tissue damage and summarizing the major lesions in an attempt to compare and put them in relation, also with human infections. Some information on pathogenesis and genetic diversity is also included. Investigating the lesions and distribution of CoVs can be crucial to understand and monitor the evolution of these viruses as well as of other pathogens and to further deepen the pathogenesis and transmission of this disease to help public health preventive measures and therapies.
Keywords: One Health; coronavirus; pathology; veterinary medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

