Natural-Based Hydrogels for Tissue Engineering Applications
- PMID: 33322369
- PMCID: PMC7763437
- DOI: 10.3390/molecules25245858
Natural-Based Hydrogels for Tissue Engineering Applications
Abstract
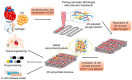
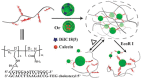
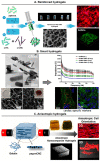
In the field of tissue engineering and regenerative medicine, hydrogels are used as biomaterials to support cell attachment and promote tissue regeneration due to their unique biomimetic characteristics. The use of natural-origin materials significantly influenced the origin and progress of the field due to their ability to mimic the native tissues' extracellular matrix and biocompatibility. However, the majority of these natural materials failed to provide satisfactory cues to guide cell differentiation toward the formation of new tissues. In addition, the integration of technological advances, such as 3D printing, microfluidics and nanotechnology, in tissue engineering has obsoleted the first generation of natural-origin hydrogels. During the last decade, a new generation of hydrogels has emerged to meet the specific tissue necessities, to be used with state-of-the-art techniques and to capitalize the intrinsic characteristics of natural-based materials. In this review, we briefly examine important hydrogel crosslinking mechanisms. Then, the latest developments in engineering natural-based hydrogels are investigated and major applications in the field of tissue engineering and regenerative medicine are highlighted. Finally, the current limitations, future challenges and opportunities in this field are discussed to encourage realistic developments for the clinical translation of tissue engineering strategies.
Keywords: DNA; anisotropy; biomimetic; blood derivatives; decellularized tissue; extracellular matrix; glycosaminoglycans; nanoparticles; proteins; supramolecular crosslinking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


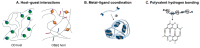
References
-
- Mano J., Silva G., Azevedo H., Malafaya P., Sousa R., Silva S., Boesel L., Oliveira J., Santos T., Marques A., et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

