Leveraging 3D Model Systems to Understand Viral Interactions with the Respiratory Mucosa
- PMID: 33322395
- PMCID: PMC7763686
- DOI: 10.3390/v12121425
Leveraging 3D Model Systems to Understand Viral Interactions with the Respiratory Mucosa
Abstract
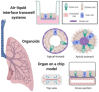
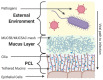
Respiratory viruses remain a significant cause of morbidity and mortality in the human population, underscoring the importance of ongoing basic research into virus-host interactions. However, many critical aspects of infection are difficult, if not impossible, to probe using standard cell lines, 2D culture formats, or even animal models. In vitro systems such as airway epithelial cultures at air-liquid interface, organoids, or 'on-chip' technologies allow interrogation in human cells and recapitulate emergent properties of the airway epithelium-the primary target for respiratory virus infection. While some of these models have been used for over thirty years, ongoing advancements in both culture techniques and analytical tools continue to provide new opportunities to investigate airway epithelial biology and viral infection phenotypes in both normal and diseased host backgrounds. Here we review these models and their application to studying respiratory viruses. Furthermore, given the ability of these systems to recapitulate the extracellular microenvironment, we evaluate their potential to serve as a platform for studies specifically addressing viral interactions at the mucosal surface and detail techniques that can be employed to expand our understanding.
Keywords: 3D model; microscopy; mucosal barrier; mucus; periciliary layer; tissue engineering; viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
In Vitro Modelling of Respiratory Virus Infections in Human Airway Epithelial Cells - A Systematic Review.Front Immunol. 2021 Aug 18;12:683002. doi: 10.3389/fimmu.2021.683002. eCollection 2021. Front Immunol. 2021. PMID: 34489934 Free PMC article.
-
Authentic Modeling of Human Respiratory Virus Infection in Human Pluripotent Stem Cell-Derived Lung Organoids.mBio. 2019 May 7;10(3):e00723-19. doi: 10.1128/mBio.00723-19. mBio. 2019. PMID: 31064833 Free PMC article.
-
Effects of paramyxoviral infection on airway epithelial cell Foxj1 expression, ciliogenesis, and mucociliary function.Am J Pathol. 2001 Dec;159(6):2055-69. doi: 10.1016/S0002-9440(10)63057-X. Am J Pathol. 2001. PMID: 11733356 Free PMC article.
-
A toolbox for studying respiratory viral infections using air-liquid interface cultures of human airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2021 Jul 1;321(1):L263-L280. doi: 10.1152/ajplung.00141.2021. Epub 2021 May 19. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34010062
-
Coronaviruses and the human airway: a universal system for virus-host interaction studies.Virol J. 2016 Feb 6;13:24. doi: 10.1186/s12985-016-0479-5. Virol J. 2016. PMID: 26852031 Free PMC article. Review.
Cited by
-
From Submerged Cultures to 3D Cell Culture Models: Evolution of Nasal Epithelial Cells in Asthma Research and Virus Infection.Viruses. 2021 Feb 28;13(3):387. doi: 10.3390/v13030387. Viruses. 2021. PMID: 33670992 Free PMC article. Review.
-
Influence of cell type specific infectivity and tissue composition on SARS-CoV-2 infection dynamics within human airway epithelium.PLoS Comput Biol. 2023 Aug 11;19(8):e1011356. doi: 10.1371/journal.pcbi.1011356. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37566610 Free PMC article.
-
Avian influenza A viruses exhibit plasticity in sialylglycoconjugate receptor usage in human lung cells.J Virol. 2023 Nov 30;97(11):e0090623. doi: 10.1128/jvi.00906-23. Epub 2023 Oct 16. J Virol. 2023. PMID: 37843369 Free PMC article.
-
Bioprinted Multi-Cell Type Lung Model for the Study of Viral Inhibitors.Viruses. 2021 Aug 11;13(8):1590. doi: 10.3390/v13081590. Viruses. 2021. PMID: 34452455 Free PMC article.
-
Immunofluorescence-Mediated Detection of Respiratory Virus Infections in Human Airway Epithelial Cultures.Curr Protoc. 2022 Jun;2(6):e453. doi: 10.1002/cpz1.453. Curr Protoc. 2022. PMID: 35671174 Free PMC article.
References
-
- Wu R., Yankaskas J., Cheng E., Knowles M.R., Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am. Rev. Respir. Dis. 1985;132:311–320. - PubMed
-
- Benali R., Tournier J.M., Chevillard M., Zahm J.M., Klossek J.M., Hinnrasky J., Gaillard D., Maquart F.X., Puchelle E. Tubule formation by human surface respiratory epithelial cells cultured in a three-dimensional collagen lattice. Am. J. Physiol. 1993;264:L183–L192. doi: 10.1152/ajplung.1993.264.2.L183. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

