Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors
- PMID: 33322488
- PMCID: PMC7763839
- DOI: 10.3390/pharmaceutics12121205
Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors
Abstract
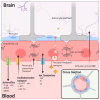
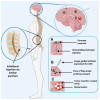
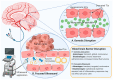
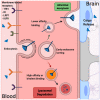
Effective treatments for brain tumors remain one of the most urgent and unmet needs in modern oncology. This is due not only to the presence of the neurovascular unit/blood-brain barrier (NVU/BBB) but also to the heterogeneity of barrier alteration in the case of brain tumors, which results in what is referred to as the blood-tumor barrier (BTB). Herein, we discuss this heterogeneity, how it contributes to the failure of novel pharmaceutical treatment strategies, and why a "whole brain" approach to the treatment of brain tumors might be beneficial. We discuss various methods by which these obstacles might be overcome and assess how these strategies are progressing in the clinic. We believe that by approaching brain tumor treatment from this perspective, a new paradigm for drug delivery to brain tumors might be established.
Keywords: BTB; NVU/BBB; blood-brain barrier; brain metastases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sarkaria J.N., Hu L.S., Parney I.F., Pafundi D.H., Brinkmann D.H., Laack N.N., Giannini C., Burns T.C., Kizilbash S.H., Laramy J.K., et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology. 2018;20:184–191. doi: 10.1093/neuonc/nox175. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

