Bovine Adenovirus-3 Tropism for Bovine Leukocyte Sub-Populations
- PMID: 33322850
- PMCID: PMC7763465
- DOI: 10.3390/v12121431
Bovine Adenovirus-3 Tropism for Bovine Leukocyte Sub-Populations
Abstract
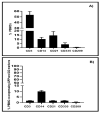
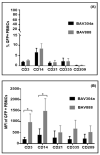
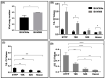
A number of characteristics including lack of virulence and the ability to grow to high titers, have made bovine adenovirus-3 (BAdV-3) a vector of choice for further development as a vaccine-delivery vehicle for cattle. Despite the importance of blood leukocytes, including dendritic cells (DC), in the induction of protective immune responses, little is known about the interaction between BAdV-3 and bovine blood leukocytes. Here, we demonstrate that compared to other leukocytes, bovine blood monocytes and neutrophils are significantly transduced by BAdV404a (BAdV-3, expressing enhanced yellow green fluorescent protein [EYFP]) at a MOI of 1-5 without a significant difference in the mean fluorescence of EYFP expression. Moreover, though expression of some BAdV-3-specific proteins was observed, no progeny virions were detected in the transduced monocytes or neutrophils. Interestingly, addition of the "RGD" motif at the C-terminus of BAdV-3 minor capsid protein pIX (BAV888) enhanced the ability of the virus to enter the monocytes without altering the tropism of BAdV-3. The increased uptake of BAV888 by monocytes was associated with a significant increase in viral genome copies and the abundance of EYFP and BAdV-3 19K transcripts compared to BAdV404a-transduced monocytes. Our results suggest that BAdV-3 efficiently transduces monocytes and neutrophils in the absence of viral replication. Moreover, RGD-modified capsid significantly increases vector uptake without affecting the initial interaction with monocytes.
Keywords: EYFP; RGD motif; bovine adenovirus-3; chimeric pIX; leukocytes; tropism.
Conflict of interest statement
The authors declare no conflict of interest. Moreover, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

