A pilot school-based health center intervention to improve asthma chronic care in high-poverty schools
- PMID: 33322963
- PMCID: PMC8281495
- DOI: 10.1080/02770903.2020.1864823
A pilot school-based health center intervention to improve asthma chronic care in high-poverty schools
Abstract
Objective: To test the feasibility and effectiveness of a multifaceted intervention administered through school-based health centers (SBHCs) to improve asthma control for children in high-poverty schools with not well controlled asthma.
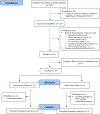
Methods: Students 4-14 years old with persistent asthma were enrolled from three SBHCs. The centers' advanced practice providers received training on evidence-based asthma guidelines. Students randomized to the intervention received directly observed therapy of their asthma controller medication, medication adjustments as needed by the centers' providers, and daily self-management support. Students randomized to usual care were referred back to their primary care provider (PCP) for routine asthma care.
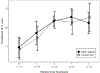
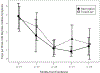
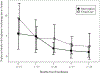
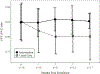
Results: We enrolled 29 students. Students in the intervention group received their controller medication 92% of days they were in school. Ninety-four percent of follow-up assessments were completed. During the study, 11 of 12 intervention students had a step-up in medication; 2 of 15 usual care students were stepped up by their PCP. Asthma Control Test scores did not differ between groups, although there were significant improvements from baseline to the 7 month follow-up within each group (both p < .01). Both FEV1% predicted and FEV1/FVC ratio significantly worsened in the usual care group (both p = .001), but did not change in the intervention group (p = .76 and .28 respectively).
Conclusions: Our pilot data suggest that a multifaceted intervention can be feasibly administered through SBHCs in communities with health disparities. Despite the small sample size, spirometry detected advantages in the intervention group. Further study is needed to optimize the intervention and evaluate outcomes.
Trial registration: clinicaltrials.gov Identifier: NCT03032744.
Keywords: Pediatrics; children; chronic care model; directly observed therapy; health disparities; health services research; medication adherence; national guidelines; prevention; pulmonary function test; spirometry; treatment.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention. Asthma: Data, Statistics, and Surveillance [4/28/20]. Available from: www.cdc.gov/asthma/asthmadata.htm
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
