Evaluation of Nutritional Gel Supplementation in C57BL/6J Mice Infected with Mouse-Adapted Influenza A/PR/8/34 Virus
- PMID: 33323164
- PMCID: PMC7754200
- DOI: 10.30802/AALAS-CM-20-990138
Evaluation of Nutritional Gel Supplementation in C57BL/6J Mice Infected with Mouse-Adapted Influenza A/PR/8/34 Virus
Abstract
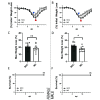
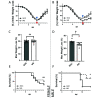
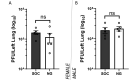
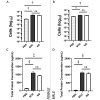
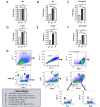
Mice are a common animal model for the study of influenza virus A (IAV). IAV infection causes weight loss due to anorexia and dehydration, which can result in early removal of mice from a study when they reach a humane endpoint. To reduce the number of mice prematurely removed from an experiment, we assessed nutritional gel (NG) supplementation as a support strategy for mice infected with mouse-adapted Influenza A/Puerto Rico/8/34 (A/PR/8/34; H1N1) virus. We hypothesized that, compared with the standard of care (SOC), supplementation with NG would reduce weight loss and increase survival in mice infected with IAV without impacting the initial immune response to infection. To assess the effects of NG, male and female C57BL/6J mice were infected with IAV at low, intermediate, or high doses. When compared with SOC, mice given NG showed a significant decrease in the maximal percent weight loss at all viral doses in males and at the intermediate dose for females. Mice supplemented with NG had no deaths for either sex at the intermediate dose and a significant increase in survival in males at the high viral dose. Supplementation with NG did not alter the viral titer or the pulmonary recruitment of immune cells as measured by cell counts and flow cytometry of cells recovered in bronchoalveolar lavage (BAL) fluid in either sex. However, mice given NG had a significant reduction in IL6 and TNFα in BAL fluid and no significant differences in CCL2, IL4, IL10, CXCL1, CXCL2, and VEGF. The results of this study show that as compared with infected SOC mice, infected mice supplemented with NG have reduced weight loss and increased survival, with males showing a greater benefit. These results suggest that NG should be considered as a support strategy and indicate that sex is an important biologic variable in mice infected with IAV.
Figures



References
-
- Barnett V, Lewis T. 1994. Outliers in statistical data, 3rd ed Chichester (England): Wiley.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

