Transcriptional readout of neuronal activity via an engineered Ca2+-activated protease
- PMID: 33323488
- PMCID: PMC7777206
- DOI: 10.1073/pnas.2006521117
Transcriptional readout of neuronal activity via an engineered Ca2+-activated protease
Abstract
Molecular integrators, in contrast to real-time indicators, convert transient cellular events into stable signals that can be exploited for imaging, selection, molecular characterization, or cellular manipulation. Many integrators, however, are designed as complex multicomponent circuits that have limited robustness, especially at high, low, or nonstoichiometric protein expression levels. Here, we report a simplified design of the calcium and light dual integrator FLARE. Single-chain FLARE (scFLARE) is a single polypeptide chain that incorporates a transcription factor, a LOV domain-caged protease cleavage site, and a calcium-activated TEV protease that we designed through structure-guided mutagenesis and screening. We show that scFLARE has greater dynamic range and robustness than first-generation FLARE and can be used in culture as well as in vivo to record patterns of neuronal activation with 10-min temporal resolution.
Keywords: neuroscience; proteases; protein engineering.
Conflict of interest statement
Competing interest statement: A.Y.T. and M.I.S. have filed a patent covering some components used in this study (U.S. provisional application 62/906,373; CZB file CZB-123S-P1; Stanford file S19-269; KT file 103182-1132922-002400PR).
Figures


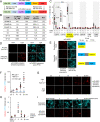
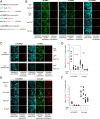

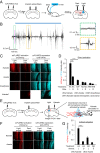
References
-
- Fields S., Song O., A novel genetic system to detect protein-protein interactions. Nature 340, 245–246 (1989). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

