CHANGES IN SUBFOVEAL CHOROIDAL THICKNESS AFTER INTRAVITREAL DEXAMETHASONE IMPLANT THERAPY FOR DIABETIC MACULAR EDEMA
- PMID: 33323903
- PMCID: PMC8140662
- DOI: 10.1097/IAE.0000000000003029
CHANGES IN SUBFOVEAL CHOROIDAL THICKNESS AFTER INTRAVITREAL DEXAMETHASONE IMPLANT THERAPY FOR DIABETIC MACULAR EDEMA
Abstract
Purpose: To investigate changes in subfoveal choroidal thickness (SFCT) and their relationship with best-corrected visual acuity and optical coherence tomography parameters after intravitreal dexamethasone implant injection for diabetic macular edema.
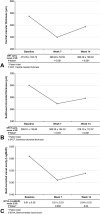
Methods: Eighty-one eyes treated with dexamethasone implant injection for diabetic macular edema were evaluated for best-corrected visual acuity, central macular thickness, SFCT, and optical coherence tomography parameters at baseline and Weeks 7 and 14.
Results: The mean baseline SFCT significantly decreased at Weeks 7 (P < 0.001) and 14 (P < 0.001). At Week 7, each 1-µm reduction in central macular thickness and five Early Treatment Diabetic Retinopathy Study letters (-0.1 logarithm of the minimal angle of resolution) improvement were associated with SFCT reductions of 0.09 (P = 0.002) and 3.91 (P = 0.044) µm, respectively. At Week 14, each 1-µm reduction in central macular thickness was associated with a 0.14-µm reduction in SFCT (P < 0.001). Eyes with good functional and anatomical responses exhibited significantly greater SFCT reductions. Subretinal fluid resulted in greater SFCT changes (P = 0.039) and better best-corrected visual acuity (P = 0.033) at Week 7. A continuous ellipsoid zone/interdigitation zone layer was associated with a smaller mean SFCT at Week 7 (P = 0.002) and better best-corrected visual acuity at Weeks 7 and 14 (both, P < 0.001).
Conclusion: Changes in SFCT after dexamethasone implant injection therapy for diabetic macular edema may predict anatomical and functional outcomes and correlate with optical coherence tomography features that are known as predictors of treatment response.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Opthalmic Communications Society, Inc.
Conflict of interest statement
None of the authors has any conflicting interests to disclose.
Figures

Comment in
-
Correspondence.Retina. 2021 Sep 1;41(9):e70. doi: 10.1097/IAE.0000000000003257. Retina. 2021. PMID: 34255762 No abstract available.
-
Reply.Retina. 2021 Sep 1;41(9):e70-e71. doi: 10.1097/IAE.0000000000003258. Retina. 2021. PMID: 34432751 Free PMC article. No abstract available.
References
-
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 2012;366:1227–1239. - PubMed
-
- van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA 2005;293:1509–1513. - PubMed
-
- Guigou S, Pommier S, Meyer F, et al. . Efficacy and safety of intravitreal dexamethasone implant in patients with diabetic macular edema. Ophthalmologica 2015;233:169–175. - PubMed
-
- Boyer DS, Yoon YH, Belfort R, Jr, et al. . Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904–1914. - PubMed
-
- Dutra Medeiros M, Postorino M, Navarro R, et al. . Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica 2014;231:141–146. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

