B cell depletion therapies in autoimmune disease: advances and mechanistic insights
- PMID: 33324003
- PMCID: PMC7737718
- DOI: 10.1038/s41573-020-00092-2
B cell depletion therapies in autoimmune disease: advances and mechanistic insights
Erratum in
-
Author Correction: B cell depletion therapies in autoimmune disease: advances and mechanistic insights.Nat Rev Drug Discov. 2025 Jan;24(1):72. doi: 10.1038/s41573-024-01103-2. Nat Rev Drug Discov. 2025. PMID: 39592853 Free PMC article. No abstract available.
Abstract
In the past 15 years, B cells have been rediscovered to be not merely bystanders but rather active participants in autoimmune aetiology. This has been fuelled in part by the clinical success of B cell depletion therapies (BCDTs). Originally conceived as a method of eliminating cancerous B cells, BCDTs such as those targeting CD20, CD19 and BAFF are now used to treat autoimmune diseases, including systemic lupus erythematosus and multiple sclerosis. The use of BCDTs in autoimmune disease has led to some surprises. For example, although antibody-secreting plasma cells are thought to have a negative pathogenic role in autoimmune disease, BCDT, even when it controls the disease, has limited impact on these cells and on antibody levels. In this Review, we update our understanding of B cell biology, review the results of clinical trials using BCDT in autoimmune indications, discuss hypotheses for the mechanism of action of BCDT and speculate on evolving strategies for targeting B cells beyond depletion.
Conflict of interest statement
J.L.G. performed consulting for Roche on anti-CD20 in multiple sclerosis in 2019 and received funding for research on the impact of anti-CD20 in experimental autoimmune encephalomyelitis (Roche) and sphingosine 1-phosphate receptor 1 inhibitors in experimental autoimmune encephalomyelitis (Novartis). These interests did not influence the content of this Review. The other authors declare no competing interests.
Figures

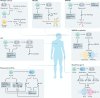

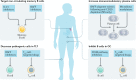
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

